��Ŀ����
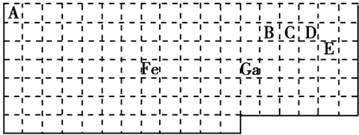
3��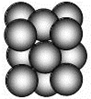 ��������Ԫ�أ�����B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش�����
��������Ԫ�أ�����B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش�����| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� I1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F ��ǰ������ԭ�ӵ��ӹ����ʾʽ�е�����������Ԫ�� |
| G�����ڱ��ĵ�ʮһ�� |
��2��C��������������Ԫ��ԭ�ӵĵ�һ�������ɴ�С��˳����Mg��Al��Na��
��3����DE3����ԭ�ӵ��ӻ���ʽΪsp3�ӻ����÷����еļ��DZ�����DE4+�еļ���С��ԭ���ǹµ��Ӷ���ɼ����ӶԵ��ų����������ڳɼ����Ӷ�֮����ų�������
�ڽ�E�ĵ���ͨ�뵽��Ѫ��{K4[Fe��CN��6]}��Һ�У��ɵõ���Ѫ��{K3[Fe��CN��6]}���÷�Ӧ�����ӷ���ʽΪ2[Fe��CN��6]4-+Cl2=2[Fe��CN��6]3-+2Cl-
����֪��E��һ�ֻ�������������ⷢ�����·�Ӧ������ƽ����H2O2+
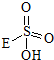 ��
��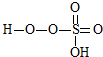 +HE����д�����������ʵĽṹʽ��
+HE����д�����������ʵĽṹʽ����4��Fλ��d�����۵����Ų�ʽΪ3d54s1��
��5��G���ʾ�����ԭ�ӵĶѻ���ʽΪ�����������ܶѻ�����ͼ��������ÿ��ԭ�ӵ���λ��Ϊ12�����辧���߳�Ϊa��ԭ�Ӱ뾶Ϊr����ʽ��ʾ�þ����Ŀռ�������Ϊ$\frac{\sqrt{2}}{6}��$��
���� B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ��������������
BԪ��ԭ�ӵĺ���p��������s��������1��B��2�����Ӳ㣬Ϊ1s22s22p3����BΪNԪ�أ�
��Cԭ�ӵĵ�һ�����ĵ��������ݿ�֪�����������ܾ�������C����+2�ۣ����ڢ�A�壬ԭ����������NԪ�أ���CΪMgԪ�أ�
D���ڵ������ڣ�Dԭ�Ӻ�������p���ȫ���������������Ų�Ϊ3s23p3����DΪPԪ�أ�
E���ڵ������ڣ�EԪ�ص������������������IJ�Ϊ4��E���ڵڢ�A�壬��EΪClԪ�أ�
F��������Ԫ���У���Χ�����Ų�Ϊndxnsy�����ܼ����ڰ����ȶ�״̬ʱ�����е�δ�ɶԵ�������࣬��FΪCrԪ�أ�
G�ڵ����������ڱ��ĵ�ʮһ�У�GΪCuԪ�أ��ݴ˽���С�⼴�ɣ�
��� �⣺���ݷ�����֪��BΪN��CΪMg��DΪP��EΪCl��FΪCr��GΪCu��
��1��BΪNԪ�أ���������Ų�ʽΪ1s2ns2np3����̬ԭ����������ߵĵ��ӣ�����2p�ܼ�����3�����ӣ���������ڿռ���3������ԭ�ӹ���ʷĴ��Σ��ʴ�Ϊ��3���Ĵ���
��2��CΪþ����MgԪ�����ڵ�ͬ����Ԫ��ΪNa��Al��ͬһ�����ڣ�����ԭ�����������ӣ�ԭ�Ӱ뾶��С��ϡ��������⣩��ԭ�Ӻ˶������ӵ�����Խ��Խǿ��Ԫ�ص�ԭ��Խ��Խ��ʧ���ӣ����Ԫ�صĵ�һ�����ܳ���������ƣ����ڢ�AԪ�صĵ�һ�����ܴ��ڵڢ�A��ģ����Ե�һ�����ܴ�С��ϵΪ��Mg��Al��Na��
�ʴ�Ϊ��Mg��Al��Na��
��3����DE3ΪPCl3��PCl3��Pԭ�ӹµ��Ӷ���Ϊ1����3���Ҽ�����Ϊsp3�ӻ���DE4+ΪNH4+�����������д���һ�Թµ��Ӷԣ��µ��Ӷ���ɼ����ӶԵ��ų����������ڳɼ����Ӷ�֮����ų����������ʰ����м��Ǵ���笠��м��ǣ��ʴ�Ϊ��sp3���µ��Ӷ���ɼ����ӶԵ��ų����������ڳɼ����Ӷ�֮����ų���������
��E�ĵ���Ϊ��������ӦΪΪ�����ͻ�Ѫ�Σ�������Ϊ��Ѫ�Σ��ʴ˷�Ӧ�����ӷ�Ӧ����ʽΪ��[Fe��CN��6]4-+Cl2=2[Fe��CN��6]3-+2Cl-���ʴ�Ϊ��[Fe��CN��6]4-+Cl2=2[Fe��CN��6]3-+2Cl-��
���������ķ���ʽ��֪���˷�ӦΪ˫��ˮ�е�H��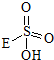 ��ȥ��E�IJ���ȡ�����ɵģ������ɵ���һ�ֲ���Ľṹ��ʽΪ��
��ȥ��E�IJ���ȡ�����ɵģ������ɵ���һ�ֲ���Ľṹ��ʽΪ��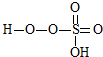 ���ʴ�Ϊ��
���ʴ�Ϊ��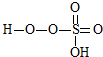 ��
��
��4��FΪCrԪ�أ���25��Ԫ�أ�λ�ڵ������ڵ�VB�壬�������Ϊd���ӣ�Ϊd��Ԫ�أ��۵����Ų�ʽΪ3d54s1���ʴ�Ϊ��d��3d54s1��
��5����ͼ��֪������Ϊ���������ѻ����Զ����Cuԭ�ӷ�����λ�����ĵ�Cuԭ����֮���������1������ԭ��Ϊ12���湲�ã�����λ��Ϊ12��Cuԭ�Ӱ뾶Ϊr���Խ���������ԭ�����ڣ��Խ��߳���=4r�����ⳤ=$\sqrt{\frac{��4r��^{2}}{2}}$=2$\sqrt{2}$r���������=��2$\sqrt{2}$r��3���þ�����Alԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�������ܶ�=$\frac{m}{V}$=$\frac{\frac{M}{{N}_{A}}��4}{��2\sqrt{2}r��^{3}}$g/cm3=$\frac{\sqrt{2}M}{8{N}_{A}{r}^{3}}$g/cm3��
ÿ��Cuԭ�����=$\frac{4}{3}$��r3���þ����к���4��Alԭ�ӣ����Ծ���������Cuԭ�����=4��$\frac{4}{3}$��r3���ռ�������=$\frac{����ͭԭ�����}{�������}$=$\frac{4��\frac{4}{3}��{r}^{3}}{��2\sqrt{2}r��^{3}}$=$\frac{\sqrt{2}��}{6}$��
�ʴ�Ϊ��12�� $\frac{\sqrt{2}��}{6}$��
���� ���⿼�����ʽṹ�����ʣ��漰Ԫ�������ɡ��ӻ��������������Ų��������ṹ�����ȣ�ע��Ե�����ͻ������⣬����������Ҫѧ������һ������ѧ����������ռ����������Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | 1-��ϩ��1��3-����ϩ | B�� | ����ͻ����� | ||
| C�� | ���غ�����泥�NH4CNO�� | D�� | 1��4-���ױ���1��3��5-���ױ� |
| A�� | NH4+ | B�� | HCO3- | C�� | SO42- | D�� | Mg2+ |
�������ӻ������ֻ�������Ӽ���û�й��ۼ���
�ڽ����ͷǽ���ֻ�ܻ����γ����Ӽ���
�����Ӽ��������ӡ������ӵ��������
����PCl5��CCl4�и�ԭ���������ܴﵽ8���ӵ��ȶ��ṹ��
�ݹ��ۼ�ֻ�����ڹ��ۻ������У�
��������˵����ȷ���ǣ�������
| A�� | �٢ڢ���ȷ | B�� | ������ȷ | ||
| C�� | ����ȷ����������ȷ | D�� | ���ٲ���ȷ |
| A�� | 1��2-������ϩClHC=CHCl | B�� | 1��1-������ϩ Cl2C=CH2 | ||
| C�� | ��ϩ CH3-CH=CH2 | D�� | ����ϩ |
| A�� | H2O | B�� | KCl | C�� | NaOH | D�� | HCl |