��Ŀ����
13��N��O��Na��Mg��S��Br�dz���������Ԫ�أ���1��Brλ��Ԫ�����ڱ��������ڵ�VIIA�壻Na��O�γ�1��1�Ļ�����ĵ���ʽ��
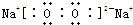 �������ʺ��еĻ�ѧ��Ϊ�����Ӽ��ͷǼ��Լ���
�������ʺ��еĻ�ѧ��Ϊ�����Ӽ��ͷǼ��Լ�����2���á�����������գ�
| �ȶ��� | ���Ӱ뾶 | �۵� | ���ļ��� |
| NH3��H2O | O2-�� Mg2+ | SO3�� Na2O2 | H-O�� H-N |
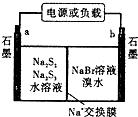
�ٷŵ�ʱNa+��b��Ǩ�ƣ��a��b��������
�ڷŵ�ʱ�����ĵ缫��ӦʽΪ��3S22--2e-=2S32-
��4����֪N4Ϊ��������ṹ��N-N����Ϊ167kJ•mol-1��N��N����Ϊ942kJ•mol-1��д��N4��g��ת��ΪN2��g�����Ȼ�ѧ����ʽN4��g��=2N2��g����H=-882kJ/mol��
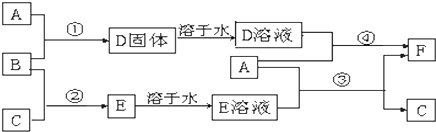
���� ��1������Ԫ��ԭ�ӵ��Ӳ���������������ȣ�����������������������ȣ��ݴ��ж�Br�����ڱ��е�λ�ã�Na��O�γ�1��1�Ļ������ǹ������ƣ����������д������Ӽ��ͷǼ��Լ���
��2��Ԫ�صķǽ�����Խǿ�����⻯����ȶ���Խǿ�����Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С�����Ӿ����۷е���ڷ��Ӿ��壻�ǽ�����Ԫ�طǽ�����Խǿ����������������Խǿ������ļ���Խǿ��
��3���ٷŵ�ʱ������ӷ�����ԭ��Ӧ����a�Ǹ�����b���������������Һ���������������ƶ���
�ڷŵ�ʱ��������ʧ���ӷ���������Ӧ��
��4���÷�Ӧ�ʱ�=��Ӧ�����-��������ܣ��ݴ���д�Ȼ�ѧ����ʽ��
��� �⣺��1������Ԫ��ԭ�ӵ��Ӳ���������������ȣ�����������������������ȣ�Brԭ�Ӻ�����4�����Ӳ㡢������������7������λ�ڵ������ڵ�VIIA�壻Na��O�γ�1��1�Ļ������ǹ������ƣ������ʽΪ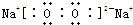 �����������д������Ӽ��ͷǼ��Լ���
�����������д������Ӽ��ͷǼ��Լ���
�ʴ�Ϊ���ģ�VIIA��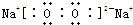 �����Ӽ��ͷǼ��Լ���
�����Ӽ��ͷǼ��Լ���
��2��Ԫ�صķǽ�����Խǿ�����⻯����ȶ���Խǿ���ǽ�����N��O�������ȶ���NH3��H2O��
���Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С���������Ӱ뾶O2-��Mg2+��
���Ӿ����۷е���ڷ��Ӿ��壬���������Ƿ��Ӿ��塢�������������Ӿ��壬�����۵�SO3��Na2O2��
�ǽ�����Ԫ�طǽ�����Խǿ����������������Խǿ������ļ���Խǿ���ǽ�����O��N������ļ���H-O��H-N��
�ʴ�Ϊ������������������
��3���ٷŵ�ʱ������ӷ�����ԭ��Ӧ����a�Ǹ�����b���������������Һ���������������ƶ���������b�缫�ƶ����ʴ�Ϊ��b��
�ڷŵ�ʱ��������ʧ���ӷ���������Ӧ���缫��ӦʽΪ3S22--2e-=2S32-���ʴ�Ϊ��3S22--2e-=2S32-��
��4���÷�Ӧ�ʱ�=��Ӧ�����-���������=6��167kJ/mol-2��942kJ/mol=-882kJ/mol����÷�Ӧ�Ȼ�ѧ����ʽΪN4��g��=2N2��g����H=-882 kJ/mol���ʴ�Ϊ��N4��g��=2N2��g����H=-882 kJ/mol��
���� ���⿼��λ�ýṹ�������ϵ��Ӧ�ã��漰�Ȼ�ѧ����ʽ����д��ԭ���ԭ����Ԫ�������ɡ�Ԫ�����ڱ��ṹ��֪ʶ�㣬�ѵ��ǵ缫��Ӧʽ����д���ʱ����ͨ�����ܻ�Ӧ�Ƚ��м��㣬��Ŀ�ѶȲ���

 �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д� ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д�| A�� | NH+4��NO3��K+��SO2-4 | B�� | Fe3+��HCO3-��Cl-��Na+ | ||
| C�� | Al��OH��-4��CO2-3��K+��Cl- | D�� | SO2-4��Na+��Cu2+��NO-3 |
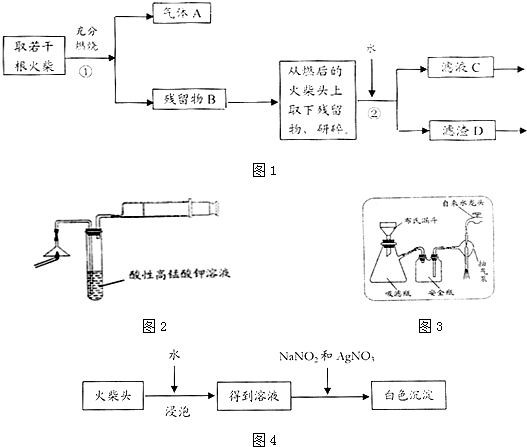
��ش��������⣺
��1��Ϊ��֤����A������ͼ2��ʾ����ʵ�飺���ܹ۲쵽KMnO4��Һ���Ϻ�ɫ����ɫ��������֤�����ͷ�Ϻ���SԪ�أ�
��2��д��������з�����Ӧ�Ļ�ѧ����ʽ2KClO3$\frac{\underline{MnO_2}}{��}$2KCl+O2����S+O2$\frac{\underline{\;��ȼ\;}}{\;}$SO2��
��3������ڵ�ʵ�����װ����ͼ3��ʾ���ò����������Ǽ�ѹ���ˣ�����ˣ���
��4��Ҫ֤�����ͷ�к���ClԪ�صĺ���ʵ�鲽����ȡ��ҺC������HNO3��AgNO3��Һ�����۲쵽��ɫ��������������֤�����ͷ�к�����Ԫ�أ�
��5����ѧ�����������ͷ��KClO3��һ��ʵ�鷽������ͼ4��ʾ�йص����ӷ�Ӧ����ʽΪClO3-+5Cl-+6H+�T3Cl2��+3H2O��Cl2+2I-�TI2+2Cl-������������������г��ְ�ɫ���������ܳ��˵�����ͷ��KClO3�Ĵ��ڣ���������MnO2��Ũ���Ṳ��Ҳ�ɲ�������
��6����С��²�����D��˫��ˮ�ֽ������������ʻ����һ����Ӱ�죬��Ʋ�����������5��ʵ�飮
| ʵ�� ���� | H2O2��Һ ��������% | H2O2��Һ����/����] | ����D ����/�� | ��Ӧ �¶�/�� | �ռ��������/���� | ���� ʱ��/�� |
| �� | 30 | 5 | 0 | 85 | 2 | 3.8 |
| �� | 15 | 2 | 0.1 | 20 | 2 | 2.8 |
| �� | 15 | 2 | 0.2 | 20 | 2 | 2.2 |
| �� | 5 | 2 | 0.1 | 20 | 2 | 7.4 |
| �� | 30 | 5 | 0 | 55 | 2 | 10.5 |
֤������D������Խ��Ӧ����Խ�죮
| A�� | ��������C12��ȼ�� | B�� | ͭ��������ȼ�� | ||
| C�� | ������������ȼ�� | D�� | ����������ȼ�� |
| A�� | Cu2+��NO3-��Cl-��Na+ | B�� | NH4+��Mg2+��NO3-��SO42- | ||
| C�� | K+��Ca2+��HCO3-��Cl-�� | D�� | Cl-��SO42-��K+��Na+ |