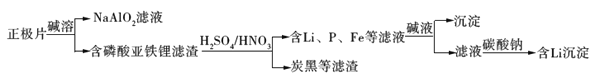
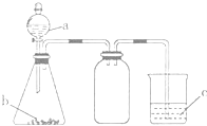
��Ŀ����
����Ŀ���Ժ�������(��Ҫ����Fe2O3��FeO��NiO��SiO2��)Ϊԭ�ϣ���ȡ��ˮ����������[NaFe3(SO4)2(OH)6]���������۵IJ��ֹ����������£�

��֪��Fe3+��pHԼΪ3.7ʱ����ȫת��ΪFe(OH)3��Fe2+��pHԼΪ9ʱ����ȫת��ΪFe(OH)2
��1����ҵ�ϳ��������ˢ�������Һ������ϴ��Һ�ϲ�����Ŀ����____��
��2�������������̷��������ӷ���ʽΪ___��
��3���������������м���̼���Ƶ�����Һ��pH��2��̼���ƹ���ᵼ�����ɵij����ɻ�������ת��Ϊ_____(�ѧʽ)��
��4���������ˢ���������Һ(����Ni2��)�м���N2H4��H2O���ڲ�ͬŨ�ȵ�����������Һ�з�Ӧ�����������XRDͼ����ͼ��ʾ(XRDͼ�������ж�ij��̬�����Ƿ���ڣ���ͬ��̬���ʳ�������������Dz�ͬ)�����Ƶøߴ��������������˵�NaOH��Ũ��Ϊ____��д�����������Ʊ���������ͬʱ����N2�����ӷ���ʽ��_____��

��5����ͬNaOHŨ���£�����Ni�ĺ�����ͬ�����ܵ�ԭ����_____��
���𰸡�Ϊ���������Ԫ�ص������� 2H����2Fe2����ClO��=2Fe3����Cl����H2O Fe(OH)3 0.015mol��L��1 N2H4��H2O��2Ni2����4OH��=2Ni����N2����5H2O ����Խǿ��N2H4��H2O�Ļ�ԭ��Խǿ
��������
��������(��Ҫ����Fe2O3��FeO��NiO��SiO2��)�������������������������Һ�к���Ni2+������������Fe2+��Fe3+�ȣ������к��ж������裻����NaClO��������������Ϊ�����ӣ�����̼������Һ������Һ��pH��ʹ������ȫ���������õ���ˮ���������������˺����Һ���ټ���̼���Ƴ��������ӵ�NiCO3����NiCO3�������ɵõ�Ni���ݴ˷������
(1)��ҵ�ϳ��������ˢ�������Һ(����Ni2+������������Fe2+��Fe3+)������ϴ��Һ�ϲ��������������Ԫ�ص������ʣ��ʴ�Ϊ�����������Ԫ�ص������ʣ�
(2)����NaClO��������������Ϊ�����ӣ���Ӧ�����ӷ���ʽΪ2H++2Fe2++ClO-�T2Fe3++Cl-+H2O���ʴ�Ϊ��2H++2Fe2++ClO-�T2Fe3++Cl-+H2O��
(3)̼���ƹ��࣬��Һ���Թ�ǿ����ᵼ�����ɵij����ɻ�������ת��ΪFe(OH)3���ʴ�Ϊ��Fe(OH)3��
(4)��ͼ���֪������������Ũ��Ϊ0.015 molL-1ʱ�����Ƶøߴ��������ۣ�����ʽΪN2H4H2O+2Ni2++4OH-�T2Ni��+N2��+5H2O���ʴ�Ϊ��0.015 molL-1��N2H4H2O+2Ni2++4OH-�T2Ni��+N2��+5H2O��
(5)����(4)�ķ�Ӧ��֪��N2H4��H2O���л�ԭ�ԣ�����Խǿ��N2H4H2O�Ļ�ԭ��Խǿ�����²�ͬNaOHŨ���£�����Ni�ĺ�����ͬ���ʴ�Ϊ������Խǿ��N2H4H2O�Ļ�ԭ��Խǿ��

 ��У����ϵ�д�
��У����ϵ�д�