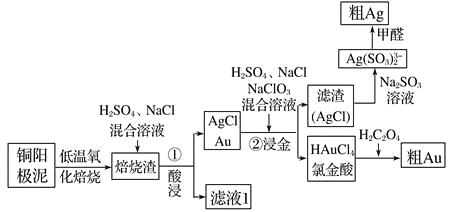
��Ŀ����
����Ŀ����������ѧ��ѧ�г��������壬����;�㷺��
��1����ҵ�ϰ��������������͵����ϳɡ�
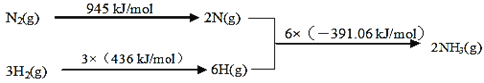
�ٸ÷�Ӧ���Ȼ�ѧ����ʽ��___��
��450�棬��5molN2��5molH2����2L�����ܱ������з�����Ӧ��5min��ﵽƽ�⣬N2��ת����Ϊ20%����NH3�Ļ�ѧ��Ӧ����v(NH3)=___�����¶�����ͼ��ʾ��Ӧ��ƽ�ⳣ������ֵK=___���¶����ߣ�ƽ�ⳣ��K��___������������������С����������������
��2����ҵ�������β���к��϶��SO2��Ϊ��ֹ��Ⱦ��������������SO2����ҵ�ϳ��ð�ˮ���շ�����β����
�ٵ���ˮ�������������ʵ���Ϊ3mol�����ձ�״����44.8LSO2ʱ����Һ�е�����Ϊ___��
����֪NH4HSO3��Һ�����ԡ��ð�ˮ����SO2��������Һ������ʱ����Һ������Ũ�ȹ�ϵ��ȷ����___��
A.c(NH4+)=2c(SO32-)+c(HSO3-)
B.c(NH4+)>c(SO32-)>c(H+)=c(OH-)
C.c(NH4+)+c(H+)=c(SO32-)+c(HSO3-)+c(OH-)
D.c(NH4+)+c(NH3��H2O)=c(SO32-)+c(HSO3-)+c(H2SO3)
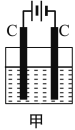
��3��������һ�ָ���ȼ�ϣ�����ֱ������ȼ�ϵ�أ���ͼ�ǹ���ˮʽȼ�ϵ�ع���ԭ����
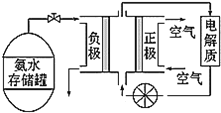
�ٰ���ȼ�ϵ�صĵ������Һ���ѡ��___������������������������������������Һ��
�ڰ���ȼ�ϵ�صķ�Ӧ�ǰ�������������һ�ֳ������������ˮ���õ�ص��ܷ�Ӧ����ʽΪ___�������ĵ缫��ӦʽΪ___��
���𰸡�N2(g)+3H2(g)=2NH3(g) ��H=-93.36kJ/mol 0.2mol��L-1��min-1 0.5 ��С (NH4)2SO3��NH4HSO3 AB ���� 4NH3+3O2=2N2+6H2O O2+4e-+2H2O=4OH-����3O2+12e-+6H2O=12OH-��
��������
(1)���ȸ�����H=��Ӧ����ܺ�-��������ܺ������H������д�Ȼ�ѧ����ʽ���ڽ�ϻ�ѧƽ������ʽ��ʽ���㣻
(2)�ٰ������ʵ���Ϊ3mol����״����44.8L SO2�����ʵ���Ϊ2mol���������պ���Һ��Nԭ�Ӻ�Sԭ�ӵĹ�ϵ���(NH4)2SO3��NH4HSO3�Ļ�ѧʽ�����жϣ������õ���غ㡢�����غ����Һ������Է����жϣ�
(3)�ٰ����Ǽ������壻�����ɵ�������ʱN2���ݴ���д�ܷ�Ӧ������������������ԭ��Ӧ����ϼ��Ի�����д������Ӧʽ��
(1)�١�H=��Ӧ�����֮��-���������֮��=945kJ/mol+3��436kJ/mol-6��391.06kJ/mol =-93.36 kJ/mol�����ԣ����Ȼ�ѧ����ʽΪ��N2(g)+3H2(g)2NH3(g) ��H=-93.36 kJ/mol���ʴ�Ϊ��N2(g)+3H2(g)2NH3(g)��H=-93.36 kJ/mol��
��450�棬2L�ܱ������У�5mol N2��5mol H2������Ӧ���ﵽƽ��ʱ��N2��ת����Ϊ20%.

NH3�Ļ�ѧ��Ӧ����v(NH3)=![]() = 0.2mol��L-1��min-1��ƽ�ⳣ��K=
= 0.2mol��L-1��min-1��ƽ�ⳣ��K=![]() =0.5�����¶��·�Ӧ��ƽ�ⳣ������ֵ��0.5���÷�ӦΪ���ȷ�Ӧ���¶����ߣ�ƽ�������ƶ���ƽ�ⳣ��K����С���ʴ�Ϊ��0.2mol��L-1��min-1��0.5����С��
=0.5�����¶��·�Ӧ��ƽ�ⳣ������ֵ��0.5���÷�ӦΪ���ȷ�Ӧ���¶����ߣ�ƽ�������ƶ���ƽ�ⳣ��K����С���ʴ�Ϊ��0.2mol��L-1��min-1��0.5����С��
(2)�ٰ������ʵ���Ϊ3mol�����ձ�״����44.8L(2mol) SO2ʱ����Һ�к���Nԭ��3mol��Sԭ��2mol������ԭ���غ㣬��(NH4)2SO3�У���ԭ�Ӹ�����Ϊ2��1����NH4HSO3�У���ԭ�Ӹ�����Ϊ1��1�����ԣ���������(NH4)2SO3��NH4HSO3���ʴ�Ϊ��(NH4)2SO3��NH4HSO3��
��NH4HSO3��Һ�����ԣ��ð�ˮ����SO2��������Һ������ʱ��c(H+)=c(OH-)�����ݵ���غ㣺c(NH4+)+c(H+)=2c(SO32-)+c(HSO3-)+c(OH-)����c(NH4+)=2c(SO32-)+c(HSO3-)����A��ȷ��C������c(NH4+)>c(SO32-)>c(H+)=c(OH-)����B��ȷ��NH4HSO3��Һ�����ԣ�������Һ�����ԣ�˵����NH4HSO3�Ͱ�ˮ�Ļ����Һ�����������غ㣬c(NH4+)+c(NH3��H2O)��c(SO32-)+c(HSO3-)+c(H2SO3)����D���ʴ�Ϊ��AB��
(3)�ٰ����Ǽ������壬���Ե��Һ���ѡ�������Һ���ʴ�Ϊ�����ԣ�
�����ɵ���������N2����ѧ����ʽΪ��4NH3+3O2=2N2+6H2O������������������ԭ��Ӧ�������ĵ缫��ӦʽΪ��O2+4e-+2H2O=4OH-���ʴ�Ϊ��4NH3+3O2=2N2+6H2O��O2+4e-+2H2O=4OH-��

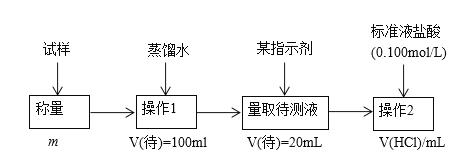
����Ŀ��ij�о���ѧϰС����Ũ��Ϊ0.20 mol��L��1�������Һ�ζ�����һ�������ʵ��ռ���Ʒ(���������Ӧ)����ˮ�γɵ���Һ��
(1)ȷ��ȡһ������Ĵ���Һ��Ҫʹ�õ�������________________��
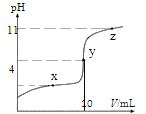
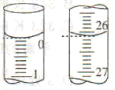
(2)���ζ���ʼ�ͽ���ʱ���ζ����е�Һ����ͼ��ʾ�������������Һ�����Ϊ______mL��
(3)�������Һ�ζ������ռ���Ʒ����Һʱ��________(������������������������ͬ)����ʽ�ζ��ܵĻ�����________ҡ����ƿ���۾�ʼ��ע��________________��
(4)�ζ�ʱ�����Է�̪Ϊָʾ�����ζ��ﵽ�յ�ı�־��__________��
(5)��ȷ��ȡ��5.0g�ռ���Ʒ���Ƴ�250mL����Һ�����������Һ�ζ����ζ�ǰ�������ζ�����������ʾ��
�ζ����� | ����Һ���(mL) | 0.20mol/L���������(mL) | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 10.00 | 0.70 | 20.60 |
�ڶ��� | 10.00 | 4.00 | 24.10 |
������ | 10.00 | 1.10 | 21.10 |
��ʵ�����ݿ�֪���ռ�Ĵ���Ϊ________��
(6)���в����ᵼ�²�õĴ���Һ��Ũ��ƫ�����________(����)��
a�����ֱ�Һ�γ���ƿ��
b���ô�����Һ��ϴ��ƿ
c����ƿϴ������������ˮ
d���ų���Һ�ĵζ��ܿ�ʼ�����ݣ��ų�Һ���������ʧ