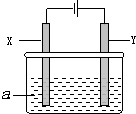
��Ŀ����
6�����Ļ����������������е�Ӧ�ù㷺��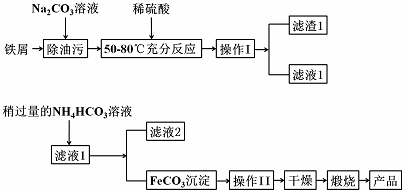
��1��������أ�K2FeO4������ǿ�����ԣ���һ������Чˮ������������ˮ���������У���Ԫ�ر���ԭΪ+3 �ۣ�������������K2FeO4����ǿ�����ԣ���ɱ����������ԭ���������ˮ�����ʣ�
��2������������Ҫ��ҵ���ϣ��÷���м�Ʊ�����������ͼ����ش��������⣺
�ٲ���������Ʒֱ��ǹ��ˡ�ϴ�ӣ�
�ڲ��� I ������Һ����������Խ�ǿ�ģ�NH4��2CO3 ��Һ��������� Fe��OH��2�����ܵ�ԭ���ǣ�FeCO3��s��+2OH-��aq��?Fe��OH��2��s��+CO32-��aq�����÷�Ӧ��ƽ�ⳣ��K=4��104 ������ֵ����
��֪��Ksp��FeCO3��=3.2��10-11��Ksp��Fe��OH��2��=8.0��10-16
��д���ڿ���������FeCO3�����������Ʒ�Ļ�ѧ����ʽ4FeCO3+O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+4CO2��
�������������֣���Ʒ�н���Fe2+ ���ڣ������Ʒ������Fe2+��ʵ�������ȡ������Ʒ���������Թ��У���������ϡ���ᣬ���Թܣ��ټ��뼸�ε���KMnO4��Һ������Һ��ɫ֤������Fe2+�������ڣ�
��3���� Cr2O72-�����Է�ˮ�ɼ���FeSO4����ת��Ϊ����Cr3+����Ӧ�����ӷ���ʽΪ6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O��
���� ��1��������ؾ���ǿ�����ԣ��仹ԭ������������������������ԣ�
��2���ٷ��������Ͳ������ù��ˣ���ȥ�����ϵ�����������������ϴ�ӣ�
�ڸ���K=$\frac{c��C{{O}_{3}}^{2-}��}{{c}^{2}��O{H}^{-}��}$=$\frac{\frac{{K}_{sp}��FeC{O}_{3}��}{c��F{e}^{2+}��}}{\frac{{K}_{sp}[Fe��{OH��}_{2}]}{c��F{e}^{2+}��}}$���м��㣻
��FeCO3��������Ӧ���������������Ͷ�����̼��
���������շ�Ӧ����ȫ�������������к���̼�������������ȼ����ܽ⣬�ټ�����������Һ�����������ӵĴ��ڣ�
��3��FeSO4���л�ԭ�ԣ�����Cr2O72-+����������ԭ��Ӧ����Fe3+��Cr3+��
��� �⣺��1���ڴ���ˮ�����У�������ر���ԭ���������ӣ�������ˮ�����������������壬������������ԣ������ܾ�ˮ��������ؾ���ǿ�����ԣ���ɱ��������������������K2FeO4����ǿ�����ԣ���ɱ����������ԭ���������ˮ�����ʣ�
�ʴ�Ϊ��K2FeO4����ǿ�����ԣ���ɱ����������ԭ���������ˮ�����ʣ�
��2���ٷ��������Ͳ������ù��ˣ���ȥ�����ϵ�����������������ϴ�ӣ�
�ʴ�Ϊ�����ˣ�ϴ�ӣ�
����FeCO3��s��+2OH-��aq��?Fe��OH��2��s��+CO32-��aq������֪��K=$\frac{c��C{{O}_{3}}^{2-}��}{{c}^{2}��O{H}^{-}��}$=$\frac{\frac{{K}_{sp}��FeC{O}_{3}��}{c��F{e}^{2+}��}}{\frac{{K}_{sp}[Fe��{OH��}_{2}]}{c��F{e}^{2+}��}}$=$\frac{3.2��1{0}^{-11}}{8.0��1{0}^{-16}}$=4��104��
�ʴ�Ϊ��4��104��
���ڿ���������FeCO3��FeCO3��������Ӧ���������������Ͷ�����̼����Ӧ����ʽΪ��4FeCO3+O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+4CO2��
�ʴ�Ϊ��4FeCO3+O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+4CO2��
�������������֣���Ʒ�н���Fe2+ ���ڣ���Ҫ��Fe2O3��FeCO3��ȡ��Ʒ�����Թ��У�����ϡ�����ܽ�õ���Һ��������������Һ��������ĸ��������Һ��ɫ֤�������������ӣ������ڣ�
�ʴ�Ϊ��ȡ������Ʒ���������Թ��У���������ϡ���ᣬ���Թܣ��ټ��뼸�ε���KMnO4��Һ������Һ��ɫ֤������Fe2+�������ڣ�
��3��FeSO4���л�ԭ�ԣ�����Cr2O72-+����������ԭ��Ӧ����Fe3+��Cr3+����Ӧ�����ӷ���ʽΪ��6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O��
�ʴ�Ϊ��6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O��
���� ���⿼�������Ļ���������ʼ���Ӧ�ã���Ŀ�Ѷ��еȣ��漰��ˮԭ������ѧʵ���������������Ӧ�á��������ܽ�ȼ��㡢���ӷ���ʽ��д��֪ʶ������֪ʶ��϶࣬��ֿ���ѧ���ķ�����������ѧʵ�顢��ѧ����������

| A�� | ˮ���������۹��� | B�� | �����Ȼ�þ | ||
| C�� | �Ҵ�����ˮ | D�� | ������ʯ |
| A�� | п����ϡ���ᷴӦ��Zn+2H+=Zn2++H2�� | |
| B�� | �廯����Һ��������Ӧ��Br-+Cl2=Cl-+Br2 | |
| C�� | ������ͭ��ϡ���ᷴӦ��OH-+H+=H2O | |
| D�� | ����ˮ��Ӧ��Na+2H2O=Na++2OH-+H2�� |
| A�� | Fe2+��Na+��Cl-��SCN- | B�� | Na+��Cu2+��NO3-��Cl- | ||
| C�� | Na+��H+��NO3-��SO42- | D�� | Al3+��Na+��Cl-��NH3•H2O |
| A�� | ��Ӧ2NO2�TN2O4�������¿��Է����У���÷�Ӧ�ġ�H��0 | |
| B�� | ��ҵ�ϵ������̬�Ȼ���ұ�������� | |
| C�� | CH3COOH ��Һ��ˮϡ�ͺ���Һ��$\frac{c��C{H}_{3}COOH��}{c��C{H}_{3}CO{O}^{-}��}$��ֵ���� | |
| D�� | Na2CO3��Һ�м�������Ca��OH��2���壬CO32-ˮ��̶ȼ�С����Һ��pH ��С |
| A�� | ���Ӱ뾶��O2-��Na+��Mg2+��Al3+��F- | |
| B�� | ���ȶ��ԣ�HCl��H2S��PH3��AsH3 | |
| C�� | ����ǿ����H2SiO3��H2CO3��H3PO4��H2SO4 | |
| D�� | ����ǿ����KOH��NaOH��Mg��OH��2��Al��OH��3 |
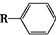 $��_{һ������}^{CO��HCl}$
$��_{һ������}^{CO��HCl}$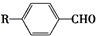 ��Ϊ�ϳ�ij��Һ�����ϵ��м���M������������²�ͬ�ĺϳ�;����
��Ϊ�ϳ�ij��Һ�����ϵ��м���M������������²�ͬ�ĺϳ�;����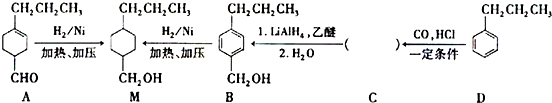
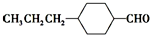 ��
�� ��д�ṹ��ʽ�����ɣ�
��д�ṹ��ʽ�����ɣ�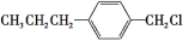 ��
�� ��C��H2O
��C��H2O ��D��Na2O2
��D��Na2O2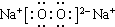 ��
��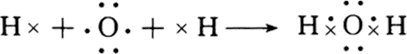 ��MgCl2
��MgCl2 ��
��