��Ŀ����
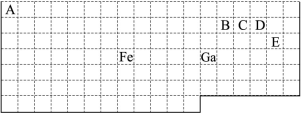
A��B��C��D��E��F���ֶ���������Ԫ�أ�ԭ��������������
| Ԫ�� | ��Ϣ |
| B | �䵥���ڳ�����Ϊ˫ԭ�ӷ��ӣ���A���γɷ� ��X��X��ˮ��Һ�ʼ��� |
| D | �����������X������ͬ������������ͬ�� ���м��������а뾶��С�� |
| E | Ԫ��ԭ�������ȴ������2������ |
| C��F | ����Ԫ�ص�ԭ������㹲��13������ |
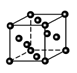
��(1)B��ԭ�ӽṹʾ��ͼΪ ��
(2)B��C��E�ֱ���A�γɵĻ����������ȶ����� (д��ѧʽ)��
(3)C��D�γɵĻ�����Ļ�ѧʽ�� ���������ʾ������������ʵķ�����Ӧ���� ����д���û������Ӧˮ�������ķ���ʽ ��
(4)F�ĵ����ڷ�Ӧ�г��� �����õ��ʵ�ˮ��Һ��E�ĵͼ������ﷴӦ�����ӷ���ʽ ��
(1) 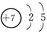
(2)H2O
(3)Al2O3������������ Al3����3OH�� Al(OH)3
Al(OH)3 AlO2-��H����H2O
AlO2-��H����H2O
(4)������Cl2��SO2��2H2O=4H����2Cl����SO42-
����

��ϰ��ϵ�д�
 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д�
�����Ŀ
�±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���Ԫ�ط��Ż�ѧʽ�ش��������⣺
| ���� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| �� | | | | | �� | | �� | |
| �� | �� | �� | �� | �� | | | �� | �� |
| �� | �� | | | | | | �� | |
��1������ЩԪ���У���������ǿ��Ԫ����
��2����ѧ��������õ�Ԫ����ԭ�ӽṹʾ��ͼΪ ��
��3��Ԫ�ص�����������Ӧ��ˮ������������ǿ���� ��������ǿ���� �������Ե����������� ��
��4���ڢۡ���Ԫ���У�ԭ�Ӱ뾶������ ��ԭ�Ӱ뾶��С���� ��
��5���ڢ����ĵ����У���ѧ���ʽϻ��õ��� ������ʲô��ѧ��Ӧ˵������ʵ��д����Ӧ�Ļ�ѧ����ʽ���� ��