��Ŀ����
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȡ�
| | Q | R | |
| T | | | W |
��1��T��ԭ�ӽṹʾ��ͼ��______���û�ѧ����ʽ��ʾ��ҵ����ұ��T���ʵ�ԭ����______��
��2����Wͬ�����ijԪ�أ����⻯������к���18�����ӣ��÷����д��ڵĹ� �ۼ���������______��
��3��Ԫ�صķǽ����ԣ�Q______W(�ǿ�ڡ������ڡ�)����Ϸ���ʽ������ԭ����______��
��4������R�������ͨ��״���³ʺ���ɫ������һ�Թܼף���ʹԪ��Rȫ��ת��Ϊ������������Ӧˮ���ʵ�鲽�裺��ʢ�м��Թܵ�����ˮ���У�______��
��1��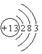 2Al2O3
2Al2O3 4Al+3O2��
4Al+3O2��
��2�����Լ��ͷǼ��Լ�
��3������ CO32����H2O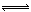 HCO3����OH�� ��̼���ˮ�⣬�������������ˮ��
HCO3����OH�� ��̼���ˮ�⣬�������������ˮ��
��4�����Թ��л���ͨ����������
��������������ɶ�����Ԫ�ؼ�ͼ��֪����Q��R�ڵڶ����ڣ�T��������������������������ȣ���T�ڵ������ڵڢ�A�壬��TΪAl�������Ƴ�QΪC��RΪN��WΪS��
��1��TΪAl��ԭ�ӽṹ����3�����Ӳ㣬����������Ϊ3��ԭ�ӽṹʾ��ͼΪ
��2����Wͬ�����ijԪ�أ����⻯������к���18�����ӣ��÷���ΪH2O2���÷��Ӵ��ڵĻ�ѧ������Ϊ�����Լ��ͷǼ��Լ���
��3������Na2CO3��Һ�����ԣ�Na2SO4��ҺΪ���ԣ�̼�ķǽ�����������ķǽ����ԣ�CO32?ˮ�ⷴӦ���ӷ���ʽΪ��CO32����H2O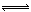 HCO3����OH�� ��̼���ˮ�⣬�������������ˮ�⡣
HCO3����OH�� ��̼���ˮ�⣬�������������ˮ�⡣
��4������R�������ͨ��״���³ʺ���ɫ��ΪNO2�����Թ��л���ͨ������������������Ӧ��4NO2+O2+2H2O=4HNO3����ȫ��ת��ΪHNO3��
���㣺���⿼��Ԫ�ص��ƶϡ���ѧ�������ԵıȽϡ����ʵ����ʡ�

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д��±��г���A��Rʮ��Ԫ�������ڱ��е�λ�ã�
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
| 2 | | | | E | R | F | |
| 3 | A | C | D | H | | I | G |
| 4 | B | | | | | | |
��ش��������⣺
��1��д��R���ʷ��ӵĵ���ʽ________��
��2��A��C��D����Ԫ�ص�����������Ӧ��ˮ�����м�����ǿ����________���ѧʽ����
��3��A��B��C����Ԫ�ص������Ӱ����Ӱ뾶�ɴ�С��˳������Ϊ________����Ԫ�ص����ӷ��ű�ʾ����
��4��д��A�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ_____________________________��
��5��XԪ����A��Rʮ��Ԫ���е�һ�֣�X��ԭ�Ӻ�����14�����ӣ�2.7 g X��������ȼ��ʱ����������2.4 g��X�������������������������Һ��Ӧ��Ҳ�������ᷴӦ��X��Ԫ�ط�����________����λ��Ԫ�����ڱ��е�________���ڵ�________�塣
�ش����¹��ڵ�������Ԫ�ؼ��仯��������⡣
��1�������������ж����ͷ��ǽ�����ǿ������ ��ѡ���ţ���
A����̬�⻯����ȶ��� B������������Ӧˮ���������
C��������������Ӧ������ D��������ͬŨ���ᷢ����Ӧ�Ŀ���
��2��Be��Al�������ƵĻ�ѧ���ʣ�д��BeCl2ˮ�ⷴӦ�Ļ�ѧ����ʽ
��3�������£������������ʷ�����Ӧ����_________������ţ�
A��CuSO4��Һ B��Fe2O3 C��Ũ���� D��NaOH E��Na2CO3����
��4����ͬѹǿ�£�����Ԫ�ط�������۵���±���
| ������ | NaF | MgF2 | SiF4 |
| �۵�/�� | 1266 | 1534 | 183 |
�Խ����ϱ��з������۵�����ԭ�� ��SiF4���ӵĿռ乹��Ϊ ��SiF4��Si��F����ļ��� ��(ѡ���ȡ���������ȡ������жϡ�)
��5��Cl2�ϳ��л���ʱ�����������HC1��4HCl+O2
 2Cl2+2H2O����ʵ���ȵ�ѭ�����á�
2Cl2+2H2O����ʵ���ȵ�ѭ�����á��÷�Ӧƽ�ⳣ���ı���ʽK= ������Ӧ�������ݻ�Ϊ2L��8min��ﵽƽ�⣬���������������2.5mol������2.25mol����HCl��ƽ����Ӧ����Ϊ mol/L? min��
W��M��N��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯����ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��M��Neԭ�ӵĺ�����������1��N��X���ڣ�X�ĵ�����һ�ֳ����İ뵼����ϣ�Y�������������������ڲ��������3����Z�ķǽ�������ͬ��������Ԫ�������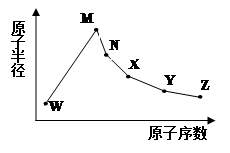
��1��Ԫ��X�����ڱ��е�λ����_______________��Ԫ��Y�����ӽṹʾ��ͼΪ____________
��2�������й��������ʵıȽ��У���ȷ����________________
| A��M��X��Z�γɵĵ����۷е� M��X��Z |
| B���⻯������ȶ��ԣ�W��X |
| C��X�ֱ���W��Z�γɵĻ������л�ѧ�����ͣ��������;���ͬ |
| D��ZԪ�غ����������һ��ǿ��YԪ�صĺ����� |
��4��X��Z��������Ԫ�ذ�1:2:2�γɵ���ԭ�ӻ���������_______���ӣ��� �����ԡ� ���Ǽ��ԡ���
��5����Zͬ���Ԫ�ض����γ��⻯�����ˮ������ǿ����______���ѧʽ��
��6������NԪ�ص����ֳ��������ܷ���˫ˮ�ⷴӦ����д�������ӷ���ʽ ________________��