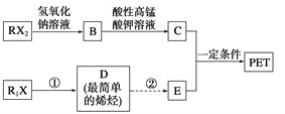
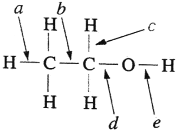
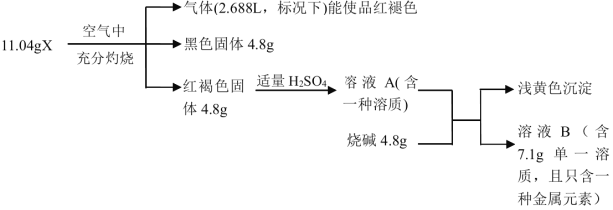
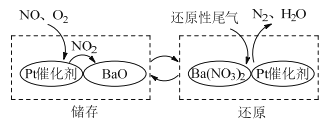
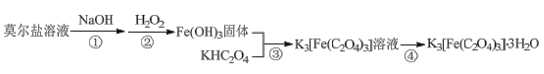
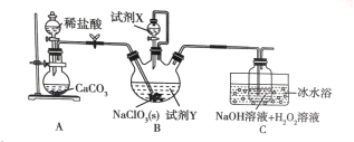
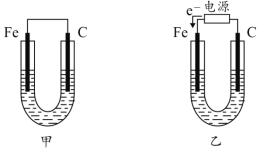
��Ŀ����
����Ŀ����1���ȽϽ�� H+���������ǿ����H2O_____NH3 ��������������������������������һ������ ����ʽ˵�� H3O+�� NH4+���� H+���������ǿ��_____��
��2��NaOCN �����ӻ������ԭ�Ӿ����� 8 �����ȶ��ṹ��д�� NaOCN �ĵ���ʽ_____��
��3����������ʱ���ⶨ�������Է��������������ݱ�����Ħ��������Ϊ 120g��mol-1���ӽṹ�� ��������ܵ�ԭ����_____
���𰸡��� H3O+��NH3��NH4+��H2O ![]() ������̬�������ͨ��������
������̬�������ͨ��������
��������
(1) ˮΪ������ʣ������ĵ�������H3O+����H3O+���ܹ��백����Ӧ����NH4+���ݴ��жϣ�
(2) NaOCN �����ӻ������ԭ�Ӿ����� 8 �����ȶ��ṹ���ݴ���д����ʽ��
(3)�������Է�������Ϊ60���ݴ˷������
(1)ˮΪ������ʣ������ĵ�������H3O+����H3O+���ܹ��백����Ӧ����NH4+����˽��H+���������ǿ����H2O��NH3 ����Ӧ�ķ���ʽΪ��H3O+��NH3��NH4+��H2O���ʴ�Ϊ������H3O+��NH3��NH4+��H2O��
(2)NaOCN �����ӻ������ԭ�Ӿ����� 8 �����ȶ��ṹ������C��N��O��ԭ�������ĵ�����������γ�8�����ȶ��ṹ��Ҫ�Ĺ��õ��Ӷ���Ŀ��֪�� NaOCN �ĵ���ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(3)��������ʱ���ⶨ�������Է��������������ݱ�����Ħ��������Ϊ 120g��mol-1���������Է�������Ϊ60��˵����������̬�������ͨ���������γ��������ӣ��ʴ�Ϊ��������̬�������ͨ�������ϡ�