��Ŀ����
18���������ữ�IJ��ᣨH2C2O4����Ԫ���ᣩ��Һ�ܽ�KMnO4��Һ�е�MnO4-ת��ΪMn2+��ij��ѧС���о����֣�����MnSO4�ɶԸ÷�Ӧ������ã�Ϊ��һ���о��й����ضԸ÷�Ӧ���ʵ�Ӱ�죬̽�����£���1���� ���£�����KMnO4��Һ��ʼŨ����ͬ�����ڲ�ͬ�ij�ʼpH�Ͳ�����Һ���������Ա�ʵ�飬�������ʵ����Ʊ���
| ʵ�� ��� | �¶� | ��ʼpH | 0.1mol/L������Һ/mL | 0.01mol/LKMnO4��Һ���/mL | ����ˮ ���/mL | �������ݣ���Ӧ���Һ��ɫʱ��/s�� |
| �� | ���� | 1 | 20 | 50 | 30 | t1 |
| �� | ���� | 2 | 20 | 50 | 30 | t2 |
| �� | ���� | 2 | 40 | 50 | 10 | t3 |
��3����t1��t2�������ʵ��ٺ͢ڵõ��Ľ�������Һ��pH�Ը÷�Ӧ��������Ӱ�죮
��4���������ʵ����֤MnSO4�Ը÷�Ӧ������ã�����±������ݣ�
| ʵ�鷽������Ҫ��д������������̣� | Ԥ��ʵ�����ͽ��� |
����Ӧ���Һ�м�������MnSO4���壬����������Ӧ������ʵ�����ͬ�����жԱ�ʵ�� | ����Ӧ���Һ��ɫʱ��С��ʵ����е�t1����MnSO4�Ը÷�Ӧ������ã�����ɫʱ����ͬ����MnSO4�Ը÷�Ӧ�����ã� |
���� ��1��ʵ��Ҫ�����KMnO4��Һ��ʼŨ����ͬ�����������Ϊ100mL������ˮ�������
��2�������������ط�Ӧ�������ӺͶ�����̼��ˮ��
��3��ʵ��ٺ͢�ֻ��pH��ͬ��������������ͬ��
��4������������Ӧ������ʵ�����ͬ���������̽��жԱ�ʵ�飻
��5�����ᷴӦ��ϣ��������һ��KMnO4��Һ����Һ��Ϊ��ɫ����ɫ30s�ڲ���ȥ��˵���ζ����յ㣻
���ݳ�����������ƽ��������Һ������ƿ����ȡҺ���õζ��ܷ�����
����������Ũ�ȣ����ɹ�ϵʽ2KMnO4��5H2C2O4���㣮
��� �⣺��1��ʵ��Ҫ�����KMnO4��Һ��ʼŨ����ͬ����KMnO4��Һ�����Ϊ50mL�������Ϊ100mL����ˮ�����10mL���ʴ�Ϊ��50��10��
��2�������������ط�Ӧ�������ӺͶ�����̼��ˮ���䷴Ӧ�����ӷ���ʽΪ��5H2C2O4+2MnO4-+6H+=10CO2��+2Mn2++8H2O��
�ʴ�Ϊ��5H2C2O4+2MnO4-+6H+=10CO2��+2Mn2++8H2O��
��3��ʵ��ٺ͢�ֻ��pH��ͬ��������������ͬ������t1��t2��˵����Һ��pH�Ը÷�Ӧ��������Ӱ�죬�ʴ�Ϊ����Һ��pH�Ը÷�Ӧ��������Ӱ�죻
��4������������Ӧ������ʵ�����ͬ���������̽��жԱ�ʵ�飬����Ӧ���Һ��ɫʱ��С��ʵ����е�t1����MnSO4�Ը÷�Ӧ������ã�����ɫʱ����ͬ����MnSO4�Ը÷�Ӧ�����ã���
�ʴ�Ϊ��
| ʵ�鷽������Ҫ��д����������̣� | Ԥ��ʵ�����ͽ��� |
| ����Ӧ���Һ�м�������MnSO4���壬����������Ӧ������ʵ�����ͬ�����жԱ�ʵ�� | ����Ӧ���Һ��ɫʱ��С��ʵ����е�t1����MnSO4�Ը÷�Ӧ������ã�����ɫʱ����ͬ����MnSO4�Ը÷�Ӧ�����ã� |
��5��������ر�������ɫ���ʲ���Ҫ����ָʾ�������ᷴӦ��ϣ��������һ��KMnO4��Һ����Һ��Ϊ��ɫ����ɫ30s�ڲ���ȥ��˵���ζ����յ㣻
����������������ƽ������250ml��Һ��250mL����ƿ����ȡKMnO4��Һ�Ͳ�����Һ�õ���ʽ�ζ��ܣ�
c�����ᣩ=$\frac{\frac{ag}{126g/mol}}{0.25L}$=$\frac{4a}{126}$mol/L��
�ɹ�ϵʽ 2KMnO4 ��5H2C2O4
2 5
cmol•L-1��V��10-3L $\frac{4a}{126}$mol/L��25��10-3mol
c=$\frac{\frac{2}{5}��\frac{4a}{126}��25��1{0}^{-3}}{V��1{0}^{-3}}$=$\frac{20a}{63V}$mol/L��
�ʴ�Ϊ���������һ��KMnO4��Һ����Һ��Ϊ��ɫ����30s�ں�ɫ����ȥ��������ƽ��250mL����ƿ������ʽ���ζ��ܣ�$\frac{20a}{63V}$��
���� ������Ҫ���ʵ�鿼����Ӱ�컯ѧ��Ӧ���ʵ����أ���Ŀ�Ѷ��еȣ�ע�������¶ȡ�Ũ�ȡ������Ի�ѧ��Ӧ���ʵ�Ӱ�죬���ö���������̽����������Է�Ӧ���ʵ�Ӱ��ʱ�����뱣֤����Ӱ�췴Ӧ���ʵ�����һ�£�

 ��ijʵ��С���H2O2�ķֽ���������̽�����±��Ǹ�ʵ��С���о�H2O2�ֽ����ʵ�����ʱ��¼��һ�����ݣ���������ͬ��״̬��ͬ��MnO2�ֱ����ʢ��15mL 5%��H2O2��Һ�Ĵ��Թ��У����ô����ǵ�ľ�����ԣ�������£�
��ijʵ��С���H2O2�ķֽ���������̽�����±��Ǹ�ʵ��С���о�H2O2�ֽ����ʵ�����ʱ��¼��һ�����ݣ���������ͬ��״̬��ͬ��MnO2�ֱ����ʢ��15mL 5%��H2O2��Һ�Ĵ��Թ��У����ô����ǵ�ľ�����ԣ�������£�| MnO2 | �����Թ���� | �۲��� | ��Ӧ��������ʱ�� |
| ��ĩ״ | ���� | ���ҷ�Ӧ�������ǵ�ľ����ȼ | 3.5min |
| ��״ | �� | ��Ӧ���������Ǻ�����ľ��δ��ȼ | 30min |
��2��ʵ���������������Ĵ�Ч����Ӵ�������йأ�
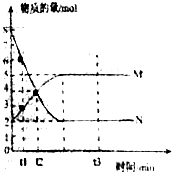
��һ���¶��£����ݻ�ΪVL���ܱ������н��з�Ӧ��aN��g��?bM��g����M��N�����ʵ�����ʱ��ı仯������ͼ��ʾ��
��1���˷�Ӧ�Ļ�ѧ����ʽ��$\frac{a}{b}$=$\frac{2}{1}$
��2��t1��t2ʱ�̣���M��Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ��$\frac{1}{��{t}_{2}-{t}_{1}��V}$mol•L-1•min-1
��3��ƽ��ʱ��N��ת����Ϊ75%��
��4��������������˵��������Ӧ�ﵽƽ��״̬����CE
A����Ӧ��M��N�����ʵ���֮��Ϊ1��1
B��������������������ʱ��ı仯���仯
C���������������ʵ�������ʱ��ı仯���仯
D����λʱ����ÿ����amolN��ͬʱ����bmolM
E����������ѹǿ����ʱ��ı仯���仯��
| A�� | ��λʱ��������n mol A��ͬʱ����2n mol C | |
| B�� | A��B��C��Ũ����� | |
| C�� | ��λʱ��������n mol A��ͬʱ����3n mol B | |
| D�� | A��B��C�ķ�����֮��Ϊ1��3��2 |
| A�� | 1mL | B�� | 4mL | C�� | 5mL | D�� | 7mL |
| A�� | ������ˮ��ʹ��ɫʯ����Һ�ȱ�����ɫ | |
| B�� | ���ʻ�����ʢ�������ļ���ƿ�У��ʻ���ɫ��˵��Cl2����Ư���� | |
| C�� | ������Ũ�����ȥ�����е�ˮ���� | |
| D�� | ������������ȼ�ղ�����ɫ����ƿ���а��� |
| A�� | ������������� | B�� | ����ϩ�ļӾ� | ||
| C�� | ���������������ˮ��Һ���� | D�� | ��ϩ���Ȼ���ӳ� |
| A�� | ԭ�Ӱ뾶�Ĵ�С˳��Ϊ��rA��rB��rC��rD��rE | |
| B�� | Ԫ��D������������Ӧ��ˮ��������Ա�E��ǿ | |
| C�� | A��D����Ԫ������Ȼ���ж�������Ӧ�ĵ��� | |
| D�� | C������������ˮ������E������������ˮ����֮�䲻�ܷ�����Ӧ |
Al4C3+12H2O��4Al��OH��3+3CH4��
Mg2C3+4H2O��2Mg��OH��2+C3H4��
������м��㣺
��1�������Ҫ��ȡ10.08L����Ȳ����״���£���������Ҫ30g CaC2����Ϊ96%�ĵ�ʯ��
��2������̼����Al4C3��Mg2C3��ɵĻ������һ������ˮ��Ӧ��ʵ���������£�
| ʵ����� | �� | �� | �� | �� |
| ̼���g�� | 7.8 | 15.6 | 23.4 | 31.2 |
| ˮ��mL�� | 18 | 18 | a | a |
| ���壨L/����� | 2.8 | 5.6 | 7 | 7 |
�ٻ������Al4C3��Mg2C3�����ʵ���֮��=1��2��
��a=22.5g��
��3������ͨ������ֽ���Ƶ���Ȳ��
����Ӧ��2CH4��C2H2+3H2������Ӧ��CH4��C��s��+2H2��
��һ��ƿ�м���Al4C3��CaC2��ɵĻ��������ձ��м���������ˮ����ַ�Ӧ����ƿ����������7.4g��ͬʱ�ռ�������8.96L������Ϊ�������ͨ������ֽ�õ��������ƽ����Է�������Ϊ11��ͬʱ��0.03g̼���ɣ��������ķֽ��ʣ�
| A�� | ��������ͨ����ˮʹ����ɫ SO2+Br2+2H2O�T4H++2Br-+SO32- | |
| B�� | ʯ��ʯ��ϡ���� CO32-+2H+�TH2O+CO2�� | |
| C�� | С�մ���Һ�еμӴ�����Һ HCO3-+CH3COOH=CH3COO-+H2O+CO2�� | |
| D�� | �Ȼ�����Һ�м�������İ�ˮ Al3++4 NH3•H2O=4NH4++AlO2-+2H2O |