��Ŀ����
2��ȫ����ÿ���������ʴ��ɴ�������ʧ��ijѧ����̽��������ˮ���Ȼ�����Һ�ʹ�����Һ��������������ʴ�Ŀ��������������ʵ�飮| ʵ����� | �� | �� | �� |
| ʵ�� ���� | 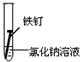 | 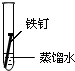 | 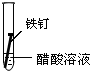 |
����һ�ܵĹ۲�����У�������ʵ�����ΪII���Թ���������ʴ�ٶ�������
�����з�ֹ������ʴ�Ĵ�ʩ����������C������ĸ����
A�������г��ĸ�Ȧ�������
B���ı�����ڲ��ṹ�Ƴɲ���֣�
C���ڵ��¸����ܵ�������ͭ��
�۳����˵�����δ��ʱϴ������Һ�к�NaCl�����ڶ�������ʴ���ֺ��ɫ��ߣ��Իش������ĸ�ʴ��Ҫ���ɵ绯ѧ��ʴ��ɵģ�
���� ��1���γ�ԭ��غ��ܼӿ�����ĸ�ʴ���ʣ�
��2�������ķ�����ʩ��������������������������ӵ���������������������е�ơ���ơ�������ȷ���ʹ�����������ˮ�����ʸ��룬�Է�ֹ������ʴ��
��3�������ĸ�ʴ��Ϊ��ѧ��ʴ�͵绯ѧ��ʴ��
��� �⣺��1�����������Ȼ�����Һ�ܹ���ԭ��أ���������������ʴ��
������ˮ�в�����������������������ˮ���ܹ���ԭ��أ�
�������ʹ����ܷ���������ԭ��Ӧ������ʴ������������ʴ�������Ǣ�
�ʴ�Ϊ��II��
��2��A�������г��ĸ�Ȧ�������������ֹ�����͵������Һ����ԭ��أ������ܷ���������ȷ��
B���ڽ����м���һЩ���������ƳɺϽ𣬸ı��˽����Ľṹ�������ܷ���������ȷ��
C������ͭ�͵������Һ����ԭ��أ�����ͭ���ã��ӿ������ĸ�ʴ�����Բ����������ã��ʴ���
�ʴ�Ϊ��C��
��3�������ĸ�ʴ��Ϊ��ѧ��ʴ�͵绯ѧ��ʴ�������˵�����δ��ʱϴ������Һ�к�NaCl�����ڶ�������ʴ���ֺ��ɫ��ߣ��������Ȼ�����Һ�ܹ���ԭ��أ���������������ʴ�����ڵ绯ѧ��ʴ���ʴ�Ϊ���绯ѧ��
���� ���⿼���˽����ĸ�ʴ�������������ԭ��Ӧ���йؼ����֪ʶ�㣬�ѶȲ����ݽ�����ʴ�ص�ѡȡ��Ӧ�ı���������

H2CrO4�THCrO${\;}_{4}^{-}$+H+
HCrO${\;}_{4}^{-}$?CrO${\;}_{4}^{2-}$+H+ Ka=3.2��10-7mol��L-1
�����ж���ȷ���ǣ�������
| A�� | 0.05 mol•L-1H2CrO4��Һ��pH=1 | |
| B�� | ��100 mL0��l mol•L-1 H2CrO4��Һ�е��뼸��NaOH��Һ����Һ��$\frac{c��Cr{O}_{4}^{2-}��}{c��HCr{O}_{4}^{-}��}$��� | |
| C�� | Na2CrO4��Һ�У�c��Na+��+c��H+��=c��CrO42-��+c��HCrO4-��+c��OH-�� | |
| D�� | ��Ũ�ȵģ�NaHCrO4��Һ��NaHCO3��Һ�У�ˮ�ĵ���̶�ǰ�ߴ� |
| A�� | 2015��3��5�գ����ǿ��������������������˵������̬���������ж������ڼ�֣�Ҫ��ȫ���ܺ�ǿ���½�3.1%���ϡ�����Դͷ��������Ⱦ������ϡ���ɫ��ѧ�������� | |
| B�� | ũҵ�����ˮ��ֲ�����ֲ������빤ҵ�л������������Ⱦ��̲��ŷḻ���������� | |
| C�� | ��������Ͼ��кܺõ��������롢������������ù���������ܣ��ѳ�Ϊ����ʱ�˵Ļ���װ���ϣ���������Ҫԭ����Ϊ�����ϣ��ɷ���SiO2��ӵ�ж�յĽṹ������ǿ������ | |
| D�� | �ѷ�ĩ״���⻯����ĭ�����ӵ����ڵĽ������У���ȴ��ɵõ�ij�ֽ�����ĭ�����øý�����ĭ����ǿ�ȵ͡�����������Կ����ڽ��캣��Ư�����У� |
| A�� | 0.1mol•L-1CH3COOH��0.1mol•L-1��ˮ�������ϣ�PH=7����c��NH4+��=c��CH3COO-��=c��H+��=c��OH-�� | |
| B�� | 0.1mol•L-1HCl��Һ��0.2mol•L-1��ˮ�������ϣ�PH��7����c��NH4+����c��Cl-����c��NH3•H2O����c��OH-�� | |
| C�� | 0.1mol•L-1CH3COONa��0.1mol•L-1CaCl2��Һ�������ϣ�c��Na+��+c��Ca2+��=c��CH3COO-��+c��CH3COOH��+2c��Cl-�� | |
| D�� | 0.1mol•L-1Na2CO3��Һ��0.1mol•L-1 NaHCO3��Һ�������ϣ�c��HCO3-����0.05mol•L-1��c��CO32-����c��OH-�� |
��1������ʵ���з�����Ӧ�����ӷ���ʽ��Zn+Cu2+=Zn2++Cu��Zn+2H+=Zn2++H2����
��2��Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬��ͬѧ���������һϵ��ʵ�飮�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧ�У��ռ����������壬��¼�����ͬ�������������ʱ�䣮
| ���� ʵ�� | A | B | C | D | E | F | |
| �� �� �� Һ | 4mol/LH2SO4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 | |
| H2O/mL | V7 | V8 | V9 | V10 | 10 | 0 | |
| ʱ�䣨min�� | 15 | 10 | 5 | 3 | 6 | 9 | |
�ڸ�ͬѧ���ó��Ľ���Ϊ������������CuSO4��Һʱ���������������ʻ�����ߣ����������CuSO4��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ����һ����������ͭ�����ɵĵ���ͭ�������Zn���棬������Zn����Һ�Ӵ��ı��棬ʹ���������������ʷ����½���
��3������п����ϡ���ᷴӦ��������������ͭ��Һ������һ��ʱ��ʣ���������壮��ͬѧ�Թ���ɷ�������м��裺
����1��ȫ��Ϊͭ��
����2������Zn����Cu��
��4���������ʵ����֤��������1������±����ݣ�
| ʵ�鷽������Ҫ��д����������̣� | Ԥ��ʵ�����ͽ��� |
| �� |
| A�� | �����Ȼ�����Һ����Һ��ɫ����������ˮ���й� | |
| B�� | NaHS��Һˮ�ⷽ��ʽΪ��HS-+H2O?H2S+OH- | |
| C�� | Na2SO3��Һ�У�c��OH-��=c��H+��+c��HSO3-��+c��H2SO3�� | |
| D�� | 0.1 mol•L-1��CH3COOH��Һ��0.1 mol•L-1��CH3COONa��Һ�������ϣ�c��CH3COO-��+c��CH3COOH��=2c��Na+�� |
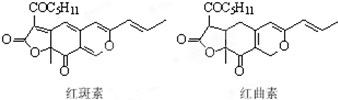 ��ɫ��ΪʹʳƷ��ɫ�����ʣ������Ӷ�ʳƷ���Ⱥü��̼�ʳ��������ء��������dz������ǹ���ѩ���ʳƷ����ɫ������Ҫ�ɷ֣��ṹ��ͼ��ʾ������˵����ȷ���ǣ�������
��ɫ��ΪʹʳƷ��ɫ�����ʣ������Ӷ�ʳƷ���Ⱥü��̼�ʳ��������ء��������dz������ǹ���ѩ���ʳƷ����ɫ������Ҫ�ɷ֣��ṹ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ����غͺ����ػ�Ϊͬ���칹�� | |
| B�� | һ�������º���غͺ����ض��ܷ����Ӿۺ����۷�Ӧ | |
| C�� | ������к����Ѽ����ʻ������������ֺ��������� | |
| D�� | 1 mol�������������6 mol H2�����ӳɷ�Ӧ |
| A�� | ���ɵ�ͭ�����ʵ�����0.2mol | B�� | ���ŵ�������Һ��pH��С | ||
| C�� | ת�Ƶ��ӵ����ʵ���Ϊ0.4mol | D�� | ������Ӧ��2H2O-4e-=4H++O2�� |
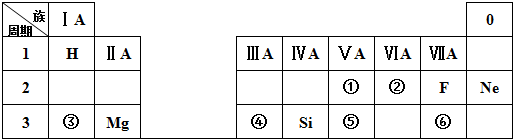
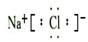 ��
��