��Ŀ����
12����֪25��ʱ��H2CrO4ˮ��Һ�д������е��룺H2CrO4�THCrO${\;}_{4}^{-}$+H+
HCrO${\;}_{4}^{-}$?CrO${\;}_{4}^{2-}$+H+ Ka=3.2��10-7mol��L-1
�����ж���ȷ���ǣ�������
| A�� | 0.05 mol•L-1H2CrO4��Һ��pH=1 | |
| B�� | ��100 mL0��l mol•L-1 H2CrO4��Һ�е��뼸��NaOH��Һ����Һ��$\frac{c��Cr{O}_{4}^{2-}��}{c��HCr{O}_{4}^{-}��}$��� | |
| C�� | Na2CrO4��Һ�У�c��Na+��+c��H+��=c��CrO42-��+c��HCrO4-��+c��OH-�� | |
| D�� | ��Ũ�ȵģ�NaHCrO4��Һ��NaHCO3��Һ�У�ˮ�ĵ���̶�ǰ�ߴ� |
���� A��H2CrO4�THCrO${\;}_{4}^{-}$+H+��˵������ĵ�һ������Ϊ��ȫ���룬0.05 mol•L-1H2CrO4��Һ����������ӵ�Ũ��Ϊ0.05mol/L������HCrO${\;}_{4}^{-}$?CrO${\;}_{4}^{2-}$+H+ Ka=3.2��10-7mol��L-1���˲�Ϊ����ȫ���룬�ʵ������ɵ�������Ũ��С��0.05mol/L���ݴ˽�ɣ�
B���������Һ�еμ�NaOH���ٽ��ڶ�������ƽ�����ƣ�c��CrO${\;}_{4}^{2-}$��Ũ������c��HCrO${\;}_{4}^{-}$��Ũ�ȼ�С���ݴ˽�ɣ�
C���κ���Һ�д��ڵ���غ㣬�ݴ˽�ɣ�
D�����ڸ���ĵ�һ��������ȫ����NaHCrO4Ϊǿ��ǿ���Σ��ݴ˽�ɣ�
��� �⣺A�����ڸ���ĵڶ���������ڵ���ƽ�⣬��Ϊ����ȫ���룬��0.05 mol•L-1H2CrO4��Һ�������������Ũ��С��0.1mol/L����pH��1����A����
B����100 mL0��l mol•L-1 H2CrO4��Һ�е��뼸��NaOH��Һ��HCrO${\;}_{4}^{-}$?CrO${\;}_{4}^{2-}$+H+ƽ�����ƣ�c��CrO${\;}_{4}^{2-}$��Ũ������c��HCrO${\;}_{4}^{-}$��Ũ�ȼ�С����Һ��$\frac{c��Cr{O}_{4}^{2-}��}{c��HCr{O}_{4}^{-}��}$���B��ȷ��
C��Na2CrO4��Һ�У�c��Na+��+c��H+��=2c��CrO42-��+c��HCrO4-��+c��OH-������CrO42-��2����λ����ɣ���ѧ������ӦΪ2����C����
D������ĵ�һ��������ȫ����NaHCrO4Ϊǿ��ǿ���Σ���NaHCrO4��ˮ�ĵ�����Ӱ�죬��NaHCO3Ϊ����ǿ���Σ��ٽ�ˮ�ĵ��룬��������ȣ�ˮ�ĵ���̶Ⱥ��ߴ�D����ѡB��
���� ������Ҫ�����������ĵ����Լ�����ƽ���Ӱ��������ƽ����ƶ����⣬ע�����׳�������Dѡ�����������Ϣ������ĵ�һ��������ȫ�жϣ���һ�����Ѷȣ�

 ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�I��ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�죮�ڳ����°������·������ʵ�飮
| ʵ���� | ��Ӧ�� | ���� |
| �� | 10mL2%��H2O2��Һ | �� |
| �� | 10mL5%��H2O2��Һ | �� |
| �� | 10mL5%��H2O2��Һ | 1mL0.1mol•L-1FeCl3��Һ |
| �� | 10mL5%��H2O2��Һ+����HCl��Һ | 1mL0.1mol•L-1FeCl3��Һ |
| �� | 10mL5%��H2O2��Һ+����NaOH��Һ | 1mL0.1mol•L-1FeCl3��Һ |
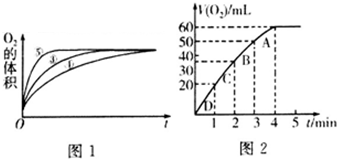
��2��ʵ��ۢܢ��У�������������������ʱ��仯�Ĺ�ϵ��ͼ1������ͼ1�ܹ��ó���ʵ������Ǽ��Ի���������H2O2�ֽ�����ʣ����Ի����ܼ�СH2O2�ֽ�����ʣ�
��3������0.1g MnO2��ĩ��50mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ2��ʾ����Ӧ���ʱ仯��ԭ����H2O2��Һ��Ũ����С����H2O2�ij�ʼ���ʵ���Ũ��Ϊ0.11mol/L��������λ��Ч���֣���
��1����ҵ����N2��H2�ϳɰ�����֪��
N2��g��+3H2��g��?2NH3��g����H=-a kJ•mol-1
2H2O��1��?2H2��g��+O2��g����H=+b kJ•mol-1
��NH3��O2����ΪN2��H2O���Ȼ�ѧ����ʽΪ4NH3��g��+3O2��g��=2N2��g��+6H2O��1����H=��2a-3b��kJ•mol-1��
��2��NH3�ܱ�H2O2���������������ˮ��
�ٴ˷�Ӧ�����ԭ��أ��ڼ��������¸����ĵ缫��Ӧ����ʽΪ2NH3+6OH--6e-=N2+6H2O��
����������ԭ��أ��ö��Ե缫���l00mL 0.5mol•L-1��CuSO4��Һ����ⷴӦ�����ӷ���ʽΪ2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu��+O2��+4H+��
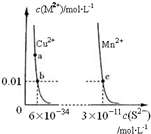
| A�� | ���¶��£�Ksp��CuS��С��Ksp��MnS�� | |
| B�� | ��CuSO4��Һ�м���MnS�������·�Ӧ��Cu2+��aq��+MnS��s��=CuS��s��+Mn2+��aq�� | |
| C�� | �ں���CuS��MnS�������Һ��c��Cu2+����c��Mn2+��Ϊ2��10-23��1 | |
| D�� | a���Ӧ�� Ksp����b���Ӧ��Ksp |
| A�� | ԭ�Ӱ뾶��W��Z��Y��X | B�� | ��̬�⻯����ȶ��ԣ�X��Z | ||
| C�� | ������������Y��W | D�� | Y��z�������ﶼ������������ |
| ʵ����� | �� | �� | �� |
| ʵ�� ���� | 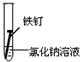 | 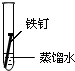 | 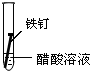 |
����һ�ܵĹ۲�����У�������ʵ�����ΪII���Թ���������ʴ�ٶ�������
�����з�ֹ������ʴ�Ĵ�ʩ����������C������ĸ����
A�������г��ĸ�Ȧ�������
B���ı�����ڲ��ṹ�Ƴɲ���֣�
C���ڵ��¸����ܵ�������ͭ��
�۳����˵�����δ��ʱϴ������Һ�к�NaCl�����ڶ�������ʴ���ֺ��ɫ��ߣ��Իش������ĸ�ʴ��Ҫ���ɵ绯ѧ��ʴ��ɵģ�
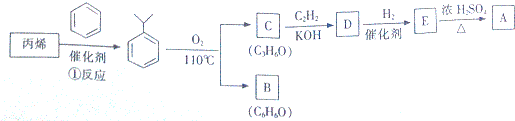
 �ճ������е��ֵ�Ͳ�ɵ��ͨ����п�̵�أ��乹��ʾ��ͼ���£�
�ճ������е��ֵ�Ͳ�ɵ��ͨ����п�̵�أ��乹��ʾ��ͼ���£� ��
��
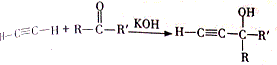
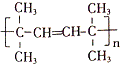 ���÷�Ӧ�������ǼӾ۷�Ӧ��B��FeCl3��Һ����ɫ��B�������DZ��ӣ�
���÷�Ӧ�������ǼӾ۷�Ӧ��B��FeCl3��Һ����ɫ��B�������DZ��ӣ�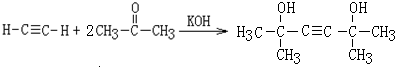 ��
��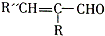 +H2O
+H2O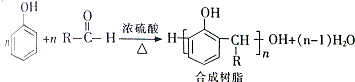
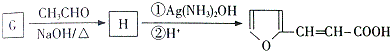
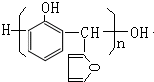 ��
��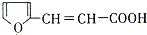 ������ͬ���칹��Ľṹ��ʽ
������ͬ���칹��Ľṹ��ʽ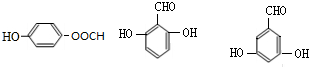 ��
��
 ��
��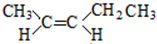 ����
����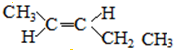 ��
�� ��
�� ����ش�
����ش�