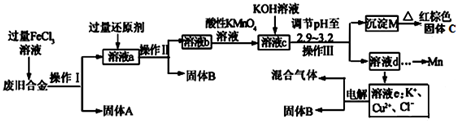
��Ŀ����
20����ѧ�����������������أ�����˵������ȷ��������Ȼ����Һ��ʯ��������Ҫ�ɷֶ��Ǽ��顡��ʯ�͵��ѽ⡢ú�����������ϻ�����ˮ��þ�Ĺ��̶�������ѧ�仯������ɫ��ѧ�ĺ�����Ӧ�û�ѧԭ���Ի�����Ⱦ������������PM2.5��ָ������ֱ��С�ڻ����2.5�Ŀ����Ҳ��Ϊ��ϸ�������PM2.5�ڿ������п����γɽ��壨������
| A�� | �� | B�� | �٢� | C�� | �ڢۢ� | D�� | �ڢ� |
���� ��Һ��ʯ������Ҫ�ɷ�ΪC4���µ�������
�������������ɵ��ǻ�ѧ�仯��
����ɫ��ѧ�ĺ����Ǵ�Դͷ�ϼ�����Ⱦ�
��С��0.1�ף�1��=1000���ף��Ŀ�����Ϊ����������������γɵķ�ɢϵ���ڽ��壮
��� �⣺��Һ��ʯ������Ҫ�ɷ�ΪC4���µ���������Ȼ������Ҫ�ɷ��Ǽ��飬�ʢٴ���
��ʯ���ѽ����ӷֽ�ΪС���ӡ�ú��������̼��ˮ��Ӧ����������һ����̼���壻���ϻ�������������Ʒ�ڼӹ��������ʹ�ù����У����ܵ��ȡ��������һ�����ص�Ӱ���������������ѧ�仯����ˮ��þͨ��������ȡ�������ᾧ�����õ�þ�������ж������л�ѧ��Ӧ���ʢ���ȷ��
������ɫ��ѧ�ĺ����Ǵ�Դͷ�ϼ�����Ⱦ������ǶԻ�����Ⱦ�����������ʢ۴���
�ܡ�PM2.5����ֱָ��С�ڵ���2.5�Ŀ��������С��0.1�ף�1��=1000���ף��Ŀ�����Ϊ����������������γɵķ�ɢϵ���ڽ��壬�ʢ���ȷ��
��ѡD��
���� ���⿼��ú��ʯ�͵��ۺ����á�������Ⱦ�����⣬ע����ɫ��ѧ����Դ���ۺ����ü��ɽ����ȷ�����Ļ�����Ⱦ�P���ܼ������������������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
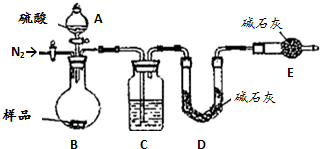
10��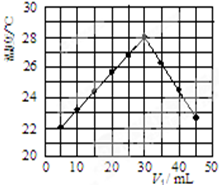 ��һ��ʵ�鷽���ⶨ��Ӧ��---------�к��Ȳⶨ
��һ��ʵ�鷽���ⶨ��Ӧ��---------�к��Ȳⶨ
��1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β�������0.5mol•L-1 ���ᡢ0.55mol•L-1NaOH��Һ����ȱ�ٵ�ʵ�鲣����Ʒ����Ͳ���¶ȼƣ�
��2�����Ǽ�¼��ʵ���������£�
��֪��Q=Cm��t2-t1������Ӧ����Һ�ı�����CΪ4.18KJ•��-1•Kg-1�������ʵ��ܶȾ�Ϊ1g•cm-3��������ɱ���H=-56.8kJ/mol
��3��ij�о�С�齫V1 mL 1.0mol/L HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ��ʾ��ʵ����ʼ�ձ���V1+V2=50mL�����ش��������⣺
�о�С������ʵ��ʱ�����¶ȵ��ڣ�����ڡ��������ڡ����ڡ���22�棬�˷�Ӧ����NaOH��Һ��Ũ��ӦΪ1.5mol/L��
������ͨ����ѧ�����ӻ��
��1����֪��1mol��H-H����I-I��H-I���ֱ���Ҫ���յ�����Ϊ436kJ��153kJ��299kJ����ӦH2��g��+I2��g��=2HI��g���ķ�Ӧ�ȡ�H=-9 kJ•mol-1
��2����֪��2H2��g��+O2��g��=2H2O ��l����H=-571.6kJ•mol-1H2��g��+1/2O2��g��=H2O��g����H=-241.8kJ•mol-1
����������Ӧȷ����H2ȼ����Ϊ285.8kJ•mol-1��
 ��һ��ʵ�鷽���ⶨ��Ӧ��---------�к��Ȳⶨ
��һ��ʵ�鷽���ⶨ��Ӧ��---------�к��Ȳⶨ��1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β�������0.5mol•L-1 ���ᡢ0.55mol•L-1NaOH��Һ����ȱ�ٵ�ʵ�鲣����Ʒ����Ͳ���¶ȼƣ�
| ʵ �� �� Ʒ | �� Һ �� �� | �к��ȡ�H | |||
| t1 | t2 | ||||
| �� | 50mL0.55mol��L-1NaOH | 50mL.0.5mol��L-1Cl | 20�� | 23.3�� | |
| �� | 50mL0.55mol��L-1NaOH | 50mL.0.5mol��L-1HCl | 20�� | 23.5�� | |
��֪��Q=Cm��t2-t1������Ӧ����Һ�ı�����CΪ4.18KJ•��-1•Kg-1�������ʵ��ܶȾ�Ϊ1g•cm-3��������ɱ���H=-56.8kJ/mol
��3��ij�о�С�齫V1 mL 1.0mol/L HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ��ʾ��ʵ����ʼ�ձ���V1+V2=50mL�����ش��������⣺
�о�С������ʵ��ʱ�����¶ȵ��ڣ�����ڡ��������ڡ����ڡ���22�棬�˷�Ӧ����NaOH��Һ��Ũ��ӦΪ1.5mol/L��
������ͨ����ѧ�����ӻ��
��1����֪��1mol��H-H����I-I��H-I���ֱ���Ҫ���յ�����Ϊ436kJ��153kJ��299kJ����ӦH2��g��+I2��g��=2HI��g���ķ�Ӧ�ȡ�H=-9 kJ•mol-1
��2����֪��2H2��g��+O2��g��=2H2O ��l����H=-571.6kJ•mol-1H2��g��+1/2O2��g��=H2O��g����H=-241.8kJ•mol-1
����������Ӧȷ����H2ȼ����Ϊ285.8kJ•mol-1��
11����NAΪ�����ӵ�������ֵ������������ȷ���ǣ�������
| A�� | ������1L 0.1 mol•L-1 NH4NO3��Һ�еĵ�ԭ����Ϊ0.2NA | |
| B�� | ��1mol H2SO4��Ũ�����������п��ȫ��Ӧ��ת�Ƶĵ�����Ϊ2NA | |
| C�� | ��״����2.24L��������к���1.9NA�Թ��õ��� | |
| D�� | ��Mg��AlΪ�缫��NaOH��ҺΪ�������Һ��ԭ����У�����������NA�����ӣ��������ų�H2�����Ϊ11.2L |
8�� ������Ԫ��W��X��Y��Z��Ԫ�����ڱ��е����λ�������ʾ��������Ԫ�ص�ԭ������������֮��Ϊ20��������˵������ȷ���ǣ�������
������Ԫ��W��X��Y��Z��Ԫ�����ڱ��е����λ�������ʾ��������Ԫ�ص�ԭ������������֮��Ϊ20��������˵������ȷ���ǣ�������
 ������Ԫ��W��X��Y��Z��Ԫ�����ڱ��е����λ�������ʾ��������Ԫ�ص�ԭ������������֮��Ϊ20��������˵������ȷ���ǣ�������
������Ԫ��W��X��Y��Z��Ԫ�����ڱ��е����λ�������ʾ��������Ԫ�ص�ԭ������������֮��Ϊ20��������˵������ȷ���ǣ�������| A�� | ����������ˮ��������ԣ�X��Z | |
| B�� | ԭ�Ӱ뾶��С��Y��X��W | |
| C�� | �⻯����ȶ���X��Y | |
| D�� | X��Y�γɵĻ����������ƻ����ǹ��ۼ� |
5�����ӵ��Լ�ƿ�������±�ʶ���京���ǣ�������


| A�� | ��ʴ�ԡ��ж� | B�� | ��ȼ��Ʒ����ȼ | C�� | ��ը�ԡ���ʴ�� | D�� | ���������ж� |
12��25��ʱ�������й���Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A�� | ������Na+��H+��OH-��CH3COO-�������ӵ�ij��Һ�д��ڣ�c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| B�� | 0.1 mol•L-1NaHCO3��Һ��0.1 mol•L-1NaOH��Һ�������ϣ�c��Na+��=2c��CO${\;}_{3}^{2-}$��+c��HCO${\;}_{3}^{-}$��+2c��H2CO3�� | |
| C�� | 0.1 mol•L-1NaHCO3��Һ��0.2 mol•L-1NaOH��Һ�������ϣ�c��Na+����c��OH-����0.05 mol•L-1��c��CO${\;}_{3}^{2-}$����c��HCO${\;}_{3}^{-}$�� | |
| D�� | pH=4.75��Ũ�Ⱦ�Ϊ0.1mol•L-1��CH3COOH��CH3COONa�����Һ��c��CH3COO-��+c��OH-����c��CH3COOH��+c��H+�� |
9������г���A��R 9��Ԫ�������ڱ��е�λ�ã�
��1����9��Ԫ�طֱ�Ϊ��дԪ�ط��ţ�ANa��BK��CMg��DAl��EC��FO��GCl��HBr��RAr�����л�ѧ��������õ���Ar��
��2��DԪ�ص�����������Ӧ��ˮ������AԪ�ص�����������Ӧ��ˮ���ﷴӦ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O��
��3��A��B��C����Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳������Ϊ��дԪ�ط��ţ�K��Na��Mg��
��4��FԪ���⻯��Ļ�ѧʽ��H2O�����⻯�ﺬ�еĻ�ѧ���ǹ��ۼ������⻯���ڳ����¸�BԪ�صĵ��ʷ�����Ӧ�Ļ�ѧ����ʽ��2K+2H2O=2KOH+H2�������⻯��ķе��ͬ��������Ԫ�ص��⻯��ķе�ߣ���ߡ��͡�����ԭ����ˮ�д��������
��5��HԪ�ظ�AԪ���γɻ�����Ļ�ѧʽ��NaBr���������ոû�����ʱ������ʻ�ɫ���õ���ʽ��ʾ�û�������γɹ��� ��
��
��6��GԪ�غ�HԪ�����ߺ˵����֮����18��G��H������������Ӧ��ˮ��������Խ������ǣ�д��ѧʽ��HBrO4��
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | E | F | ||||||
| 3 | A | C | D | G | R | |||
| 4 | B | H |
��2��DԪ�ص�����������Ӧ��ˮ������AԪ�ص�����������Ӧ��ˮ���ﷴӦ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O��
��3��A��B��C����Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳������Ϊ��дԪ�ط��ţ�K��Na��Mg��
��4��FԪ���⻯��Ļ�ѧʽ��H2O�����⻯�ﺬ�еĻ�ѧ���ǹ��ۼ������⻯���ڳ����¸�BԪ�صĵ��ʷ�����Ӧ�Ļ�ѧ����ʽ��2K+2H2O=2KOH+H2�������⻯��ķе��ͬ��������Ԫ�ص��⻯��ķе�ߣ���ߡ��͡�����ԭ����ˮ�д��������
��5��HԪ�ظ�AԪ���γɻ�����Ļ�ѧʽ��NaBr���������ոû�����ʱ������ʻ�ɫ���õ���ʽ��ʾ�û�������γɹ���
 ��
����6��GԪ�غ�HԪ�����ߺ˵����֮����18��G��H������������Ӧ��ˮ��������Խ������ǣ�д��ѧʽ��HBrO4��