��Ŀ����
����Ŀ��NiCl2�ǻ����ϳ�������Ҫ����Դ����ҵ�����ú���(Ni)�ϴ�������Ҫ����Ni��������SiO2��Al2O3��Fe�������������ᡢ������ʣ������Ȼ������壨NiCl2��nH2O��������ͼ��
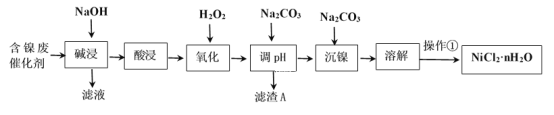
���ֽ�����������Ksp����ֵ���±���ʾ��
��ѧʽ | Fe(OH)2 | Fe(OH)3 | Al(OH)3 | Ni(OH)2 |
Ksp����ֵ | 10-17 | 10-38 | 10-34 | 10-15 |
�ش��������⣺
��1��Al��ԭ�ӽṹʾ��ͼΪ___��
��2��������������ܽ�����ʹ�õ���Ϊ___���������ʱ������Ӧ�����ӷ���ʽΪSiO2+2OH-=SiO32-+H2O��____��
��3��������������H2O2��Һ����������___(�����ӷ���ʽ��ʾ)��Ȼ�����pHʹ��Һ����Ԫ��ǡ����ȫ����������Ũ����10-5mol��L-1ʱ�����ӳ�����ȫ������ʱ�����µ�pHԼΪ____��
��4��������������ʵ���������Ϊ�������ȣ�Ũ����___Ϊֹ����ȴ�ᾧ�����ˡ�ϴ�ӡ�������ò�Ʒ��
��5���������ѳ�Ϊ��϶�����������Ҫ������ͣ����ڼ��Ե������Һ�Ĺ���ԭ�����£�M+Ni(OH)2![]() MH+NiOOH(ʽ��MΪ����Ͻ�)��д����س������������ĵ缫��Ӧʽ___��
MH+NiOOH(ʽ��MΪ����Ͻ�)��д����س������������ĵ缫��Ӧʽ___��
���𰸡� ���� Al2O3+2OH-=2AlO2-+H2O 2Fe2++H2O2+2H+=2Fe3++2H2O 3 ��Һ������ֽᾧ��Ĥ Ni(OH)2+OH--e-=NiOOH+H2O
���� Al2O3+2OH-=2AlO2-+H2O 2Fe2++H2O2+2H+=2Fe3++2H2O 3 ��Һ������ֽᾧ��Ĥ Ni(OH)2+OH--e-=NiOOH+H2O
��������
ij����(Ni)�ϴ�������Ҫ����Ni��������SiO2��Al2O3��Fe�������������ᡢ������ʣ��������̵�Ŀ�����ú����ϴ����Ʊ�NiCl2nH2O���壬�����������̣�����Ni�ϴ�����NaOH��Һ���ݣ�SiO2��Al2O3����NaOH���ɿ�����ˮ��Na2SiO3��NaAlO2�����й��˲�������������ΪFe��Ni�����������ڼ�����ʣ��ٽ����������Fe��Ni�������γ�Fe2+��Ni2+�����й��˲�������������������ʱ����˳�����Һ����Ҫ����Fe2+��Ni2+����H2O2������Һ�е�Fe2+����Fe3+���ټ��� Na2CO3��Һ������ҺpH��ʹFe3+��ȫת��ΪFe(OH)3���������˺���Һ���ټ����μ�Na2CO3��Һ����Ni(OH)2����������������Ni(OH)2�����������ܽ⣬������Ũ������ȴ�ᾧ�����˼���NiCl2nH2O���壬�ݴ˷�����
(1)Al��ԭ������Ϊ13��λ��Ԫ�����ڱ��������ڣ�������3�����Ӳ㣬������������Ϊ3������ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(2)���������̵�Ŀ�����ú����ϴ����Ʊ�NiCl2nH2O���壬��������������ܽ�����ʹ�õ���Ϊϡ����������ʱSiO2��Al2O3����NaOH���ɿ�����ˮ��Na2SiO3��NaAlO2��������Ӧ�����ӷ���ʽΪSiO2+2OH-=SiO32-+H2O��Al2O3+2OH-=2AlO2-+H2O���ʴ�Ϊ�����Al2O3+2OH-=2AlO2-+H2O��
(3)��Fe3+�����γ�Fe(OH)3�ﵽȥ��FeԪ�ص�Ŀ�ģ������H2O2��Һ��Ŀ��������Fe2+ΪFe3+�����������ӷ�ӦΪ2Fe2++H2O2+2H+=2Fe3++2H2O������pHʹ��Һ����Ԫ��ǡ����ȫ���������Է�����������Һ��c(Fe3+)Ũ�ȵ���10-5mol/L����ΪFe3+������ȫ����ȥ����ʱ��Һ��c(OH-)=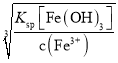 =10-11mol/L����pH =14+lgc(OH-)=3���ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��3��
=10-11mol/L����pH =14+lgc(OH-)=3���ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��3��
(4)������������Ŀ��Ϊ����Һ�л��NiCl2nH2O���壬��������Ϊ�������ȣ�Ũ������Һ������ֽᾧ��ĤΪֹ���پ���ȴ�ᾧ�����ˡ�ϴ�ӡ�������ò�Ʒ���ʴ�Ϊ����Һ������ֽᾧ��Ĥ��
(5)�������ڼ��Ե������Һ�Ĺ���ԭ�����£�M+Ni(OH)2![]() MH+NiOOH�����ʱ������Ni(OH)2����������Ӧ������NiOOH���������ĵ缫��ӦʽΪNi(OH)2+OH-- e-=NiOOH+H2O���ʴ�Ϊ��Ni(OH)2+OH--e-=NiOOH+H2O��
MH+NiOOH�����ʱ������Ni(OH)2����������Ӧ������NiOOH���������ĵ缫��ӦʽΪNi(OH)2+OH-- e-=NiOOH+H2O���ʴ�Ϊ��Ni(OH)2+OH--e-=NiOOH+H2O��

����Ŀ��ijѧ�����ڱ���������أ�(����ʽΪ![]() ��Է�������Ϊ204)�ⶨNaOH��Һ��Ũ�ȣ���NaOH��Һ��Ũ����0.1mol/L���ң��ζ��յ�ʱ��Һ��pHԼΪ9.1��
��Է�������Ϊ204)�ⶨNaOH��Һ��Ũ�ȣ���NaOH��Һ��Ũ����0.1mol/L���ң��ζ��յ�ʱ��Һ��pHԼΪ9.1��
��1��д���ڱ������������NaOH��Ӧ�Ļ�ѧ����ʽ��________��
��2�����÷�����ƽ��ȷ�������ڱ���������ط�����ƿ�У���������ˮ�ܽ⣬��Һ��ɫ���ټ���ָʾ��_____(�Ӽ��ȡ���̪��ʯ����ѡ��)����NaOH��Һ�ζ����յ�ʱ��������________��
��3����ʵ��Ҫ�õ�����Ҫ��������_______��______��
��4����ѧ����������ʵ�飬��ȡ�������������ͬ����д�±���
ʵ�� ��� | �ڱ���������ص�����(g) | ����NaOH��Һ�����(mL) |
1 | 0.4488 | 22.24 |
2 | 20.04 | |
3 | 19.96 |
�ζ������ϴ���ǵ�_____��ʵ�飬����������Ŀ���ԭ����_____
a���ζ�ʱ��NaOH��Һ�ε���ƿ���棻
b��δ��NaOH��Һ��ϴ�ζ��ܣ�
c��NaOH��Һ�ڡ�0���̶������ϣ�δ������Ϳ�ʼ�ζ���
d���۲��¼�ζ�����Һ��̶�ʱ�ζ�ǰ���ӣ��ζ����ӣ�
e����ָ̪ʾ������ɫ��Ϊ��ɫʱ����ֹͣ�ζ���
��5��NaOH��Һ�����ʵ���Ũ��Ϊ______��