��Ŀ����
14��2005��ŵ������ѧ�������ڡ�ϩ�����ֽⷴӦ���о���������������λ��ѧ�ң���ϩ�����ֽⷴӦ����ָ�ڽ����⡢�ɵȴ����������£�̼̼˫�����Ѳ�������ϵĹ��̣���������ϩ��RCH=CHR���������������û����������µ�ϩ��RCH=CHR��R��CH=CHR�䣮��C6H5CH2CH=CH2��CH2=M��һ�������»�Ϸ�Ӧ�����в��ﲻ���ܴ��ڵ��ǣ�������| A�� | C6H5CH2CH=M | B�� | CH2=CH2 | ||
| C�� | C6H5CH2CH2C6H5 | D�� | C6H5CH2CH=CHCH2C6H5 |
���� �������֪��������ΪC6H5CH2CH�TCH2��CH2�TM������˫��λ�õĶ��ѣ����Ѻ�Ļ��Ž�����������γ�˫�����ݴ˽��
��� �⣺C6H5CH2CH�TCH2����ΪC6H5CH2CH��CH2��CH2�TM����ΪCH2��M�����Ͽ�������C6H5CH2CH�TCHCH2C6H5��CH2=CH2��C6H5CH2CH�TM���ʲ���������C6H5CH2CH2C6H5����ѡ��C��
���� ������Ҫ����ѧ����ȡ��Ϣ���������Ѷ��еȣ�������Ŀ��ȡ��Ϣ�������Ӧ�����ǹؼ���

��ϰ��ϵ�д�
�����Ŀ
1�����з�ɢϵ���ȶ����ǣ�������
| A�� | ����Һ | B�� | ����Һ | C�� | ���� | D�� | ��Һ |
2���Ҵ������и��ֻ�ѧ����ͼ��ʾ�������йط�Ӧ�ϼ�λ��˵��������ǣ�������
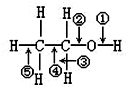

| A�� | �Ҵ������ᷢ��������Ӧʱ�ϼ��� | |
| B�� | �Ҵ��ͽ����Ƶķ�Ӧ�ϼ��� | |
| C�� | �Ҵ���Ũ���ᣬ���ȵ�170��ʱ�ϼ��ڢ� | |
| D�� | �Ҵ���Cu����������O2��Ӧʱ�ϼ��٢� |
19����NA��ʾ�����ӵ�������ֵ������������ȷ���ǣ�������
| A�� | ��״���£�22.4L�״��к��е���ԭ����Ϊ1.0NA | |
| B�� | ���³�ѹ�£�46gNO2�� N2O4�Ļ�������к��е�ԭ������Ϊ3NA | |
| C�� | ��״���£�2.24LCl2��������ϡNaOH��Һ��Ӧ��ת�Ƶ�������Ϊ0.2NA | |
| D�� | 1L 1 mol•L-1�������У������Ȼ��������ΪNA |
6�������йص������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A�� | ��0.1 mol•L-1NaHCO3��Һ�У�c��Na+����c��HCO3-����c��CO32-����c��H2CO3�� | |
| B�� | ��0.1 mol•L-1Na2CO3��Һ�У�c��OH-��-c��H+��=c��HCO3-��+2c��H2CO3�� | |
| C�� | �����£�CH3COONa��CH3COOH�����Һ[pH=7��c��Na+��=0.1 mol•L-1]��c��Na+����c��CH3COO-����c��H+��=c��OH-�� | |
| D�� | ��0.2 mol•L-1NaHCO3��Һ�м�������0.1 mol•L-1NaOH��Һ��c��CO32-����c��HCO3-����c��OH-����c��H+�� |
4����ҵ�ϳ��ð����ȡ̼���ƣ������Ͷ�����̼�ֱ��Ⱥ�ͨ�뱥��ʳ��ˮ������С�մ��پ����ˡ����գ��ô����ȴ�����ð����̼��أ�������Ϊ����Һ�У�������
| A�� | KHCO3�ܽ�Ƚϴ� | B�� | KHCO3�ܽ�Ƚ�С | C�� | K2CO3�ܽ�Ƚϴ� | D�� | K2CO3�ܽ�Ƚ�С |