��Ŀ����
����Ŀ�������ǵ���N2O����һ��ǿ�������壬����ת���ɿ�����Ⱦ��о������ǵ��ֽ�Ի�����������Ҫ���塣
��1����ˮ�����ѵ������У�������������£�����刺ɷֽ�ΪN2O����һ�ֲ���÷�Ӧ�Ļ�ѧ����ʽΪ________��
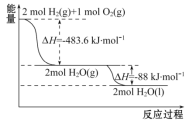
��2����֪��Ӧ2N2O(g)=2N2(g) + O2(g)�Ħ�H= �C163 kJ��mol��1��1molN2(g)��1molO2(g)�����л�ѧ������ʱ�ֱ���Ҫ����945 kJ��498 kJ����������1molN2O(g)�����л�ѧ������ʱ��Ҫ���յ�����Ϊ________ kJ��
��3����һ���¶��µĺ��������У���Ӧ2N2O(g)=2N2(g) + O2(g)�IJ���ʵ���������£�
��Ӧʱ��/min | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
C(N2O)/mol/L | 0.100 | 0.090 | 0.080 | 0.070 | 0.060 | 0.050 | 0.040 | 0.030 | 0.020 | 0.010 | 0.010 |
����0��20minʱ�Σ���Ӧ����v(N2O)Ϊ________ mol��L��1��min��1��
����N2O��ʼŨ��c0Ϊ0.150 mol��L��1����Ӧ��30minʱN2O��ת���ʦ� =__________���Ƚϲ�ͬ��ʼŨ��ʱN2O�ķֽ����ʣ�v(c0=0.150 mol��L��1) ________ v(c0=0.100 mol��L��1)���>������=����<������
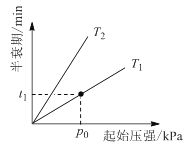
�۲�ͬ�¶ȣ�T���£�N2O�ֽ��˥������ʼѹǿ�ı仯��ϵ��ͼ��ʾ��ͼ�а�˥��ָ��һŨ��N2O����һ��ʱ�������Ӧʱ�䣩����T1________T2���>������=����<���������¶�ΪT1����ʼѹǿΪp0����Ӧ��t1 minʱ����ϵѹǿp =________����p0��ʾ����
��4�������������ܴ�������N2O�ķֽ����ʣ���Ӧ����Ϊ��
��һ�� I2(g) ![]() 2I(g) ���췴Ӧ��
2I(g) ���췴Ӧ��
�ڶ��� I(g)+N2O(g)��N2(g)+IO(g) ������Ӧ��
������ IO(g)+N2O(g)��N2(g)+O2(g)+I(g) ���췴Ӧ��
ʵ�����������ʱN2O�ֽ����ʷ���v=k��c(N2O)��[c(I2)]0.5��kΪ���ʳ����������б�����ȷ����________������)��
A��N2O�ֽⷴӦ�У�k�����⣩> k���⣩ B����һ�����ܷ�Ӧ�������������
C���ڶ�����ܱȵ������� D��I2Ũ����N2O�ֽ�������
���𰸡�NH4NO3![]() N2O+2H2O 1112.5 1.0��10-3 20.0% = > 1.25p0 AC
N2O+2H2O 1112.5 1.0��10-3 20.0% = > 1.25p0 AC
��������
��1������刺ɷֽ�ΪN2O����һ�ֲ�����������غ㶨�ɿ��ж���һ����ΪH2O�����ݵ�ʧ�����غ�д����Ӧ�Ļ�ѧ����ʽ��
��2������ӦΪ���ȷ�Ӧ����ѧ������ʱ�ų�������-��ѧ������ʱ�����յ�����=��Ӧ�ų����������Դ˷������
��3���ٸ��ݷ�Ӧ����v(N2O)=![]() ���㣻
���㣻
�ڴӱ������ݷ�����֪��ÿ��ʱ����ڣ���c(N2O)��ȣ���v(N2O)��ȣ�����ʼŨ���أ���N2O��ʼŨ��c0Ϊ0.150 mol��L��1����Ӧ��30minʱN2Oת����Ũ��Ϊ0.010mol/L��3=0.030mol/L����ת���ʦ� =![]() ��100%=20.0%��
��100%=20.0%��
���¶�Խ�߷�ӦԽ�죬��˥��Խ�̣���ͼ��֪��ѹǿһ��ʱ��T1ʱ��˥��С��T2����T1>T2������������ͬʱ��ѹǿ֮�ȵ������ʵ���֮�ȣ��ɷ�Ӧʽ2N2O(g)=2N2(g)+ O2(g)��֪������ʼʱ��2molN2O������һ��ֽ�ʱ������1molN2��0.5molO2����ʣ��1molN2O����ʱ������������ʵ�����Ϊ��1mol+1mol+0.5mol��=2.5mol������ϵѹǿp =![]() P0=1.25p0��
P0=1.25p0��
��4����ѧ��Ӧ���ʿ���ȡ���ڷ�Ӧ���������ķ�Ӧ�����ڶ�����Ӧ���÷�Ӧ���ܷ�ӦΪ2N2O(g)=2N2(g)+O2(g)�����ڷ�Ӧ����������ã��Դ˷������
��1������刺ɷֽ�ΪN2O����һ�ֲ�����������غ㶨�ɣ����ж���һ����ΪH2O����Ӧ�Ļ�ѧ����ʽΪNH4NO3![]() N2O+2H2O��
N2O+2H2O��
�ʴ�Ϊ��NH4NO3![]() N2O+2H2O��
N2O+2H2O��
��2������ӦΪ���ȷ�Ӧ����ѧ������ʱ�ų�������-��ѧ������ʱ�����յ�����=��Ӧ�ų�����������1molN2O(g)�����л�ѧ������ʱ��Ҫ���յ�����ΪxkJ����945 kJ��2+498 kJ-2x kJ =163 kJ�����x=1112.5��
�ʴ�Ϊ��1112.5��
��3������0��20minʱ�Σ�v(N2O)=![]() =
=![]() =1.0��10-3 mol��L��1��min��1��
=1.0��10-3 mol��L��1��min��1��
�ڴӱ������ݷ�����֪��ÿ��ʱ����ڣ���c(N2O)��ȣ���v(N2O)��ȣ�����ʼŨ���أ����Բ�ͬ��ʼŨ��ʱN2O�ķֽ����ʣ�v(c0=0.150 mol��L��1) = v(c0=0.100 mol��L��1)����N2O��ʼŨ��c0Ϊ0.150 mol��L��1����Ӧ��30minʱN2Oת����Ũ��Ϊ0.010mol/L��3=0.030mol/L����ת���ʦ�=![]() ��100%=20.0%��
��100%=20.0%��
�ʴ�Ϊ��20.0% ��=��
���¶�Խ�߷�ӦԽ�죬��˥��Խ�̣���ͼ��֪��ѹǿһ��ʱ��T1ʱ��˥��С��T2����T1>T2������������ͬʱ��ѹǿ֮�ȵ������ʵ���֮�ȣ��ɷ�Ӧʽ2N2O(g)=2N2(g) + O2(g)��֪������ʼʱ��2molN2O������һ��ֽ�ʱ������1molN2��0.5molO2����ʣ��1molN2O����ʱ������������ʵ�����Ϊ��1mol+1mol+0.5mol��=2.5mol������ϵѹǿp =![]() P0=1.25p0��
P0=1.25p0��
�ʴ�Ϊ��> ��1.25p0��
��4����ѧ��Ӧ���ʿ���ȡ���ڷ�Ӧ���������ķ�Ӧ�����ڶ�����Ӧ���÷�Ӧ���ܷ�ӦΪ2N2O(g)=2N2(g) + O2(g)�����ڷ�Ӧ����������ã�
A�����ڷ�Ӧ����������ã�N2O�ֽⷴӦ�У�k�����⣩> k���⣩����A��ȷ��
B���ڶ�����ӦΪ����Ӧ�����ܷ�Ӧ������������ã���B����
C���ڶ�����ӦΪ����Ӧ����������ӦΪ�췴Ӧ���ڶ�����ܱȵ�������C��ȷ��
D���ɺ���ʱN2O�ֽ����ʷ���v=k��c(N2O)��[c(I2)]0.5��֪�� N2O�ֽ�������[c(I2)]0.5�����ȣ���D����ѡAC��
�ʴ�Ϊ��AC��