��Ŀ����
����Ŀ�����ػ����Һ��Ϊȼ�ռ���Һ��Ϊ����������֪:
��H2(g)=H2(l)����H=-0. 92 kJ��mol-1 ��O2(g)=O2(l)����H=-6. 84 kJ��mol-1
����˵����ȷ����
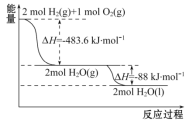
A. 2 mol H2(g)��1 mol O2(g)�������������2 mol H2O(g)�������������
B. ������ȼ����Ϊ��H=-241. 8 kJ��mol-1
C. �����Һ��ȼ�յ��Ȼ�ѧ����ʽΪ2H2(l)+O2(l)![]() 2H2O(g)����H=-474. 92 kJ��mol-1
2H2O(g)����H=-474. 92 kJ��mol-1
D. H2O(g)���H2O(l)�Ĺ����У��ϼ����յ�����С�ڳɼ��ų�������
���𰸡�C
��������
A����ͼ�������2molH2��g����1molO2��g����Ӧ����2molH2O��g�����ų�483.6kJ��������
B��������ȼ������ָ1mol������ȫȼ������Һ̬ˮ�ų���������
C����ͼ�������2H2��g��+O2��g���TH2O��l����H1=-483.6kJmol-1�٣�H2��g��=H2��l����H1=-0.92kJmol-1��O2��g��=O2��l����H2=-6.84kJmol-1������-�ڡ�2-����2���ø�˹���ɼ��㣻
D��H2O��g������H2O��l��ʱ���ų�������Ϊ�����仯������
A����ͼ�������2molH2��g����1molO2��g����Ӧ����2molH2O��g�����ų�483.6kJ����������2molH2��g����1molO2��g�������е���������2molH2O��g�������е��������ߣ���A����
B��������ȼ������ָ1mol������ȫȼ������Һ̬ˮ�ų�����������������ȼ����Ϊ��483.6+88��/2=285.8kJmol-1����B����
C����ͼ�������2H2��g��+O2��g���TH2O��l����H1=-483.6kJmol-1�٣�H2��g��=H2��l����H1=-0.92kJmol-1��O2��g��=O2��l����H2=-6.84kJmol-1������-�ڡ�2-����2�ɵ�2H2��l��+O2��l���T2H2O��g����H=-474.92kJmol-1����C��ȷ��
D��H2O��g������H2O��l��Ϊ�����仯�������ڻ�ѧ���Ķ��Ѻ����ɣ���D����
��������������ѡC��

����Ŀ��ijʵ��С���о���Һ��AgNO3��Na2S�ķ�Ӧ��
ʵ�� | �Լ� | ���� | |
| �Թ� | �ι� | |
��pH = 4�� |
��pH = 9�� | ���ֺ�ɫ���� | |
��1�������ӷ���ʽ����Na2S��ҺpH > 7��ԭ��________��
��2��ʵ��С��ͬѧ��Ϊ��ɫ�����п��ܺ���Ag2O��Ag2S��Ag�����ʵ����֤��
��֪��i��Ũ�����ܽ�Ag2Sת��Ϊ![]() ��
��![]() ��
��
ii��Ag2O���ܽ���Ũ��ˮ���γ�������Һ����Ag2S��Ag�����ܡ�
�� ��Ʋ�ʵʩ����ʵ�飬֤ʵ�����к���Ag2S��

�Լ�1���Լ�2�ֱ���_________��_________��
����1������2�ֱ���_________��_________��
�� ��Ʋ�ʵʩ����ʵ�飬֤ʵ������������Ag2O����ʵ�����������������
ʵ����� | ʵ������ | |
����i | ȡ����������Һ�������еμ����� | ���ְ�ɫ���� |
����ii | ȡ����ϴ�Ӻ�ĺ�ɫ������____________ | ____________ |
�� �����飬����������Ag��

��3��ʵ��С��ͬѧ��ΪAgNO3��Һ���������ԣ���һ���������ܹ�����Na2S�����ʵ������о���ʵ��װ������ͼ��ʾ������õ�ѹΪa��![]() ����
����
��AgNO3��Һ������![]() �����ʽ����Ʋ⣺
�����ʽ����Ʋ⣺
����1�� ![]() ��AgNO3��Һ��
��AgNO3��Һ��![]() ������
������![]() ��
��
����2�� ![]() ��AgNO3��Һ��
��AgNO3��Һ��![]() ������
������![]() ��
��
������ͼװ�ü����о�����֪����ѹ��С��ӳ������������ԭ��ǿ���IJ��죻�����������뻹ԭ��ǿ������Խ��ѹԽ��
�� ��![]() ��AgNO3��Һ�滻Ϊ_______��Һ����¼��ѹΪb��
��AgNO3��Һ�滻Ϊ_______��Һ����¼��ѹΪb��![]() ����
����
�� ����ʵ��֤ʵ������![]() ��������һ������
��������һ������![]() ����֤����______��
����֤����______��
ʵ����ۣ�AgNO3��Һ��Na2S��Һ�ķ�Ӧ�����뷴Ӧ�����йء�
����Ŀ�������ǵ���N2O����һ��ǿ�������壬����ת���ɿ�����Ⱦ��о������ǵ��ֽ�Ի�����������Ҫ���塣
��1����ˮ�����ѵ������У�������������£�����刺ɷֽ�ΪN2O����һ�ֲ���÷�Ӧ�Ļ�ѧ����ʽΪ________��
��2����֪��Ӧ2N2O(g)=2N2(g) + O2(g)�Ħ�H= �C163 kJ��mol��1��1molN2(g)��1molO2(g)�����л�ѧ������ʱ�ֱ���Ҫ����945 kJ��498 kJ����������1molN2O(g)�����л�ѧ������ʱ��Ҫ���յ�����Ϊ________ kJ��
��3����һ���¶��µĺ��������У���Ӧ2N2O(g)=2N2(g) + O2(g)�IJ���ʵ���������£�
��Ӧʱ��/min | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
C(N2O)/mol/L | 0.100 | 0.090 | 0.080 | 0.070 | 0.060 | 0.050 | 0.040 | 0.030 | 0.020 | 0.010 | 0.010 |
����0��20minʱ�Σ���Ӧ����v(N2O)Ϊ________ mol��L��1��min��1��
����N2O��ʼŨ��c0Ϊ0.150 mol��L��1����Ӧ��30minʱN2O��ת���ʦ� =__________���Ƚϲ�ͬ��ʼŨ��ʱN2O�ķֽ����ʣ�v(c0=0.150 mol��L��1) ________ v(c0=0.100 mol��L��1)���>������=����<������
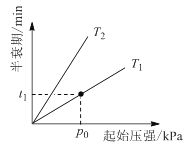
�۲�ͬ�¶ȣ�T���£�N2O�ֽ��˥������ʼѹǿ�ı仯��ϵ��ͼ��ʾ��ͼ�а�˥��ָ��һŨ��N2O����һ��ʱ�������Ӧʱ�䣩����T1________T2���>������=����<���������¶�ΪT1����ʼѹǿΪp0����Ӧ��t1 minʱ����ϵѹǿp =________����p0��ʾ����
��4�������������ܴ�������N2O�ķֽ����ʣ���Ӧ����Ϊ��
��һ�� I2(g) ![]() 2I(g) ���췴Ӧ��
2I(g) ���췴Ӧ��
�ڶ��� I(g)+N2O(g)��N2(g)+IO(g) ������Ӧ��
������ IO(g)+N2O(g)��N2(g)+O2(g)+I(g) ���췴Ӧ��
ʵ�����������ʱN2O�ֽ����ʷ���v=k��c(N2O)��[c(I2)]0.5��kΪ���ʳ����������б�����ȷ����________������)��
A��N2O�ֽⷴӦ�У�k�����⣩> k���⣩ B����һ�����ܷ�Ӧ�������������
C���ڶ�����ܱȵ������� D��I2Ũ����N2O�ֽ�������
����Ŀ����1��CO2�������⻯�ϳɵ�̼ϩ������ϳ���ϩ�ķ�ӦΪ2CO2��g��+6H2��g��![]() CH2=CH2��g��+4H2O��g����H����֪���ֻ�ѧ���������±���ʾ����CO2�Ľṹʽ��O=C=O��
CH2=CH2��g��+4H2O��g����H����֪���ֻ�ѧ���������±���ʾ����CO2�Ľṹʽ��O=C=O��
���� | H-H | C=O | C=C | C-H | H-O |
����/kJmol-1 | 436 | 745 | 615 | 413 | 463 |
���H=_____________________��
��2����ҵ�ϳ��õ����������Һ��������������ռ���Һ��װ������ͼ��ʾ������������������Ϊ���Ե缫�����ͬ��ͬѹ�£�������������ҵ������ԼΪ2:1��

������Ļ�ѧʽ____________ ��
��a�����Դ��____________ (�������������)����,�õ缫��ӦʽΪ_______________��
�����ӽ���ĤdΪ________(������ӡ��������ӡ�)����Ĥ��
�ܲ����Ϊ_________ ��Һ��