��Ŀ����
15������Ǧ���صĵ缫����Ǧ�࣬��Ҫ��PbO��PbO2��PbSO4�������Ʊ����ȶ������λ�����Ǧ����ɿɱ�ʾΪ3PbO•PbSO4•H2O������ʵ��������ͼ��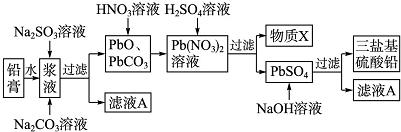
��1������X����ѭ�����ã������������ᣮ����������ʹ�õ�Na2SO3��Һ�Ƿ���ʵķ�����ȡ������Һ����������ϡ���ᣬ�ټ��Ȼ�����Һ���۲��Ƿ������ɫ������
��2������ҺA����ȡ��һ�ֺ��ᾧˮ�����θ���Ʒ��������þ����нᾧˮ�ĺ���������Ĺ����β����������������ƾ��ơ������ǡ���������ʯ�������������ȣ�
��3�������в�ֱ������H2SO4��Һ��PbO��PbCO3��Ӧ��ȡPbSO4��ԭ�������PbSO4������ˮ�������ڹ�������谭��Ӧ�Ľ�һ��������
��4���������λ�����Ǧ�����ӷ�Ӧ����ʽΪ4PbSO4+6OH-=3PbO•PbSO4•H2O+3SO42-+2H2O��
��5����Ǧ�ཬҺ�м���Na2SO3��Һ��Ŀ���ǽ����е�PbO2��ԭΪPbO����ʵ������ȡǦ�������Ϊ47.8g������PbO2����������Ϊ15.0%����Ҫ��PbO2ȫ����ԭ���������30mL 1.0mol•L-1 Na2SO3��Һ��
���� ��1�����÷Ͼ�Ǧ����������������Ǧ�ࣩ�Ʊ����ϼӹ����ȶ������λ�����Ǧʵ�����̣���Ǧ�ཬҺ�м���Na2SO3��Һ��Ŀ���ǽ�PbO2��ԭPbO��Na2SO3+PbO2=PbO+Na2SO4����Na2CO3��Һ�ǽ�PbSO4ת����PbCO3��������Һ����Ҫ��Na2SO4��Һ��PbO��PbCO3�������������ת����Pb��NO3����Pb��NO3���м�ϡH2SO4ת����PbSO4�����ᣬ���XΪHNO3����ѭ�����ã��������˵��������ᱵ������ͨ�������Ƿ�������������ӣ�
��2�����������нᾧˮ�ĺ�����ʵ�鲽��Ϊ������ĥ �ڳ�����������װ������������������ �ۼ��� ����ȴ �ݳ��� ���ظ������ݵIJ�����ֱ���������γ��������������0.1gΪֹ �߸���ʵ�����ݼ��㾧���нᾧˮ�ĺ�������������Ĺ����β��������в�������ʯ���������������������ƾ��ơ������ǣ�
��3�����ɵ�����Ǧ������ˮ�������ڹ���PbO��PbCO3�ı��棬�谭��Ӧ�Ľ�һ��������
��4�������̿�������Ǧ���������Ʒ�Ӧ�������λ�����Ǧ�������ƣ��ݴ�д������ʽ��
��5������Ǧ�������������Ǧ�����������ɼ��������Ǧ�����ʵ����������������ƺ�����Ǧ��Ӧ��ϵ�ɼ�����Ҫ�������Ƶ������
��� �⣺��1���������̿�֪��PbO��PbCO3�������������ת����Pb��NO3����Pb��NO3���м�ϡH2SO4ת����PbSO4�����ᣬ���XΪHNO3����ѭ�����ã�������������ӵķ���Ϊ��ȡ����������������ˮ��Ȼ���������ữ���ٵ�BaCl2��Һ�������ְ�ɫ��������֤���þ����к���SO42-��
�ʴ�Ϊ�����ȡ������Һ����������ϡ���ᣬ�ټ��Ȼ�����Һ���۲��Ƿ������ɫ������
��2�����������нᾧˮ�ĺ�����ʵ�鲽��Ϊ������ĥ �ڳ�����������װ������������������ �ۼ��� ����ȴ �ݳ��� ���ظ������ݵIJ�����ֱ���������γ��������������0.1gΪֹ �߸���ʵ�����ݼ��㾧���нᾧˮ�ĺ�������������Ĺ����β��������в�������ʯ���������������������ƾ��ơ������ǣ�
�ʴ�Ϊ���������ƾ��ƣ������ǣ�
��3�����ɵ�����Ǧ������ˮ�������ڹ���PbO��PbCO3�ı��棬�谭��Ӧ�Ľ�һ���������ʴ�Ϊ��PbSO4������ˮ�������ڹ�������谭��Ӧ�Ľ�һ��������
��4�������̿�������Ǧ���������Ʒ�Ӧ�������λ�����Ǧ�������ƣ���Ӧ����ʽΪ��4PbSO4+6NaOH=3PbO•PbSO4•H2O+3Na2SO4+2H2O�����ӷ���ʽΪ��
4PbSO4+6OH-=3PbO•PbSO4•H2O+3SO42-+2H2O���ʴ�Ϊ��4PbSO4+6OH-=3PbO•PbSO4•H2O+3SO42-+2H2O��
��5������Ǧ�����ʵ���Ϊ��$\frac{47.8g��15%}{239g/mol}$=0.03mol��
PbO2 ��Na2SO3
1mol 1mol
0.03mol n
n=0.03mol��V=$\frac{0.03mol}{1.0mol/L}$=0.03L=30mL��
�ʴ�Ϊ��30��
���� ���⿼����ʵ�鷽������ƣ��е��Ѷȣ�Ҫ�������̣���������������Ϣ�����Ŀ���ʽ��⣮

��1��Z������������Ӧˮ����W����Ҫ�Ļ���ԭ�ϣ�W�Ļ�ѧʽΪH2SO4����ҵ����W������������Ҫ��Ϊ�����Σ�
��101kPaʱ��3.2g Z�Ĺ��嵥����ȫȼ�տɷų�29.7kJ��������д���ܹ���ʾ�ù��嵥��ȼ���ȵ��Ȼ�ѧ����ʽS��s��+O2��g��=SO2��g����H=-297kJ/mol��
���ڽӴ������Σ�Ϊ���ZY2��ת���ʣ����������жϣ�Ӧѡ��������ǵ��º�ѹ���ӱ�������ͬ�¶ȡ�ѹǿ��ZY2ƽ��ת���ʵ�ʵ�����ݽ��з�������Ϲ�ҵ������ʵ�ʣ�Ӧѡ���ʺϵ��¶Ⱥ�ѹǿ�ǣ�ѡ����ĸ��B��
A��400�桫500��10MPa B��400�桫500��1MPa
C��500�桫500��10MPa D��400�桫500��0.1MPa
ѹǿ/MPa ת����/% �¶�/�� | 0.1 | 0.5 | 1 | 10 |
| 400 | 99.2 | 99.6 | 99.7 | 99.9 |
| 500 | 93.5 | 96.9 | 97.8 | 99.3 |
| 600 | 73.7 | 85.8 | 89.5 | 96.4 |
A��ˮ B��0.5mol/L������ C��98.3%������ D��Ũ��ˮ
��2����֪X��XY���ǹ�ҵ�ϳ��õĻ�ԭ����
��д��X������W��Ũ��Һ��Ӧ�Ļ�ѧ����ʽC+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O��
��500�棬11.2L����״����ZY2�ڴ�����������XY������ѧ��Ӧ������2��6.02��1023������ת��ʱ���÷�Ӧ�Ļ�ѧ����ʽ��SO2+2CO$\frac{\underline{����}}{��}$S+2CO2��
| A�� | SO2���������� | |
| B�� | CuFeS2������ԭ������Ԫ�ر����� | |
| C�� | ÿ����1mol Cu2S����4mol������ | |
| D�� | ÿת��1.2mol���ӣ���0.2mol������ |
��1��ʳƷ���Ӽ������NH4Al��SO4��2•12H2O���¿ɷֽ⣬���й�����ֽ�����Ԥ�ⲻ���� ����C��
A��NH3��N2��SO2��H2O B��NH3��SO3��H2O
C��NH3��SO2��H2O D��NH3��N2��SO3��SO2��H2O
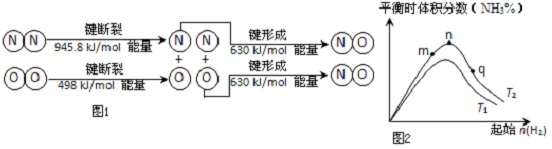
��2����������������ʱҲ������N2��O2��Ӧ����������Ⱦ��NO���������仯ʾ��ͼ��ͼ1��÷�Ӧ���Ȼ�ѧ����ʽΪN2��g��+O2��g��=2NO��g����H=+183.8kJ/mol��
��3����ҵ�ϳɰ��ķ�Ӧ��N2��g��+3H2��g��?2NH3��g����H��0���ֱ���T1��T2�¶��£��ı���ʼ�������ʵ��������ƽ��ʱ�������������ͼ2��ʾ��
�ٱȽ���m��n��q����������ƽ��״̬�У���Ӧ��N2��ת������ߵ���P�㣮
��T2�����£���2L���ܱ������У�����x mol N2�� y mol H2ʱ��3min��ƽ�⣬��ʱ��Ӧ���ת���ʾ�Ϊa��д�����н���a��x�ı���ʽ�����ػ���v��N2��=$\frac{xa}{6}$mol•L-1•min-1���÷�Ӧ��ƽ�ⳣ����ֵK=$\frac{16��xa��{\;}^{2}}{��x-xa����3x-3xa��{\;}^{3}}$��
��ͼ����T2����T1������ڡ��������ڡ��������ڡ�����ȷ��������
�ܿ�ѧ�Ҳ��ø����ӵ����Ե�SCY�մɣ��ܴ���H+ ��ʵ�ְ��ĵ绯ѧ�ϳɣ�������˵�����������ת���ʣ�д���绯ѧ�ϳɹ����з�����ԭ��Ӧ�ĵ缫����ʽ��N2+6H++6e-=2NH3��
��4����������ȵ��ķ����ۺ�ͭ�۵ľ��Ȼ����ֱ����ͬŨ��ϡ�����ַ�Ӧ������������Ļ�ԭ����ֻ��NO��ʵ���������±���
| �� �� | �� | �� | �� | �� |
| ϡ�������/mL | 100mL | 200mL | 300mL | 400mL |
| ʣ�����/g | 18.0g | 9.6g | 0 | 0 |
| NO���/L����״���£� | 2.24L | 4.48L | 6.72L | V |
A��������ʼŨ��Ϊ4mol/L B�������ܽ���5.6g Fe
C������n��Cu2+��=0.15mol D������V=6.72L
| A�� | ��֬ˮ��ɵõ���������� | |
| B�� | ����������һ���Ķ��ԣ����Բ��ܳԺ���������ʳƷ | |
| C�� | ��������Һ��������Һ�������� | |
| D�� | �����������͵����ǽ������� |
| A�� | ��������Һ�������Һ��ϣ�SiO32-+2H+=H2SiO3�� | |
| B�� | NH4Al��SO4��2��Һ�����ϡ��ˮ��Ӧ��Al3++3NH3•H2O=Al��OH��3��+3NH4+ | |
| C�� | ��ϡ������ϴ�Թ��ڱڵ�������Ag+2H++NO3-=Ag++NO2��+H2O | |
| D�� | FeBr2��Һ��ͨ�����Cl2��2Fe2++2Br-+2Cl2=2Fe3++Br2+4Cl- |
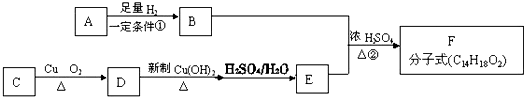
 ��
�� ��
�� ��
�� ��
��