��Ŀ����
17��A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ����������������֪����Aԭ�Ӻ���ֻ��1�����ӣ���Bԭ�������������Ǵ�����������2������Cԭ�������������ȴ�����������4������Dԭ�ӵĴ�����������������������8������E���ʼ��ܺ����ᷴӦ���ܺ��ռӦ����F��Cͬ���壮�û�ѧ���Ż�ѧ����ش��������⣺��1���õ���ʽ��ʾ��������A2C2
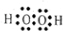 ��
����2���á���ѧʽ���͡������Ƚϣ�A��C��A��B�γɼ�������ȶ���H2O��CH4���е�ĸߵ�H2O��CH4��
��3��д��E���ռӦ�����ӷ���ʽ2Al+2OH-+2H2O=2AlO2-+3H2����
���� A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ��������������Aԭ�Ӻ���ֻ��1�����ӣ���AΪH��Bԭ�������������Ǵ�����������2����ԭ��ֻ����2�����Ӳ㣬����������Ϊ2������������Ϊ4����BΪC��Cԭ�������������ȴ�����������4����ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����CΪO��Dԭ�ӵĴ�����������������������8���������������Ϊ8������������Ϊ1����DΪNa��E���ʼ��ܺ����ᷴӦ�����ܺ��ռӦ����EΪAl��F��Cͬ���壬����FΪS���Դ������
��� �⣺A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ��������������Aԭ�Ӻ���ֻ��1�����ӣ���AΪH��Bԭ�������������Ǵ�����������2����ԭ��ֻ����2�����Ӳ㣬����������Ϊ2������������Ϊ4����BΪC��Cԭ�������������ȴ�����������4����ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����CΪO��Dԭ�ӵĴ�����������������������8���������������Ϊ8������������Ϊ1����DΪNa��E���ʼ��ܺ����ᷴӦ�����ܺ��ռӦ����EΪAl��F��Cͬ���壬����FΪS��
��1��H2O2�ĵ���ʽΪ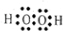 ���ʴ�Ϊ��
���ʴ�Ϊ��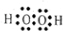 ��
��
��2��A��C�γɵĻ�����ΪH2O��A��B�γɻ�����ΪCH4���ǽ�����O��C�����ȶ��ԣ�H2O��CH4������ˮ����֮�����������ʷе㣺H2O��CH4���ʴ�Ϊ��H2O��CH4��H2O��CH4��
��3��Al���ռӦ�����ӷ�ӦΪ��2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
���� ���⿼��ԭ�ӽṹ��Ԫ�صĵ��ʡ�����������ʣ�Ԫ�ص��ƶ��ǽ����Ĺؼ���ע���Ԫ�������ɵ����⣮

| A�� | ��ʹ��ɫʯ����ֽ��������Һ�У�Na+��K+��CO32-��NO3-��AlO2- | |
| B�� | c��H+��=0.1 mol/L����Һ�У�Cu2+��Al3+��SO42-��NO3- | |
| C�� | �ڼ������ۺ��ܲ�����������Һ�У�NH4+��Fe2+��SO42-��NO3- | |
| D�� | ������OH-����Һ�У�CO32-��Cl-��F-��K+ |
 ���ֱ�ȡ�ơ��������ơ�����������ͭ����Һ�ֱ�������ʵ�����
���ֱ�ȡ�ơ��������ơ�����������ͭ����Һ�ֱ�������ʵ�����������ǡ�÷�Ӧ���ɼ��ȣ��������Ĵ��������ʵ����ʵ���֮��Ϊ��������
| A�� | 6��4��5 | B�� | 3��2��3 | C�� | 3��2��2 | D�� | 1��1��1 |
| A�� | ���� | B�� | ��ϩ | C�� | ���� | D�� | �Ҵ� |
| A�� | ըҩ��ը | B�� | ʳ�������������� | ||
| C�� | �������������ᷴӦ | D�� | Ba��OH��2•8H2O��NH4Cl��Ӧ |
Si��s����ʯӢɰ��+2C��s���TSi��s�����ֹ裩+2CO��g����H=+682.44kJ/mol
Si��s�����ֹ裩+2Cl2��s���TSiCl4��g������H=-657.01kJ/mol
SiCl4��g��+2Mg��s���T2MgCl2��s��+Si��s�������裩����H=-625.63kJ/mol
������56.00g����ķ�Ӧ��Ϊ��������
| A�� | 600.20 kJ•mol-1 | B�� | +1200.40 kJ•mol-1 | ||
| C�� | -1965.08 kJ•mol-1 | D�� | -1200.40 kJ•mol-1 |
| A�� | �����Ӿ����в����ܴ��ڷǼ��Լ� | |
| B�� | �ڹ��ۻ�����ķ��Ӿ����в����ܴ������Ӽ� | |
| C�� | �ڼ��Է����в����ܴ��ڷǼ��Լ� | |
| D�� | ��ԭ�Ӿ����в����ܴ��ڼ��Թ��ۼ� |
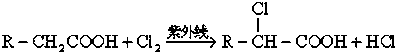 ��
��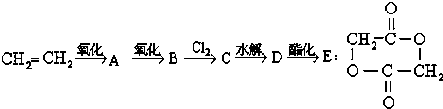
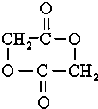 +2H2O��
+2H2O��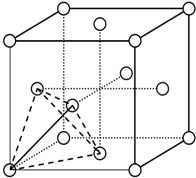 ͭ����Ҫ������Cu�Ļ������ڿ�ѧ�о���ҵ�����о���������;����CuSO4��Һ���������Һ�����Һ�ȣ���ش��������⣺
ͭ����Ҫ������Cu�Ļ������ڿ�ѧ�о���ҵ�����о���������;����CuSO4��Һ���������Һ�����Һ�ȣ���ش��������⣺