��Ŀ����
12����֪��ҵ������ϩֱ����������ȩ�����������Ȼ������ӵ�̼ԭ�ӣ���Ϊ��̼ԭ�ӣ��ϵ���ԭ�ӱ�±��ȡ������һ±���ᣬ�磺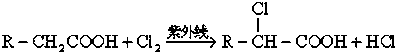 ��
��������ϩ����ԭ�Ϻϳ�E��
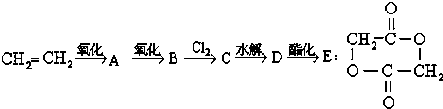
���ƶϣ�
��1��д��B��C�Ľṹ��ʽ��BCH3COOHCClCH2COOH
��2��д���йط�Ӧ�Ļ�ѧ����ʽ��
��A����������ӦCH3CHO+2Ag��NH3��OH$\stackrel{��}{��}$CH3COONH4+2Ag��+3NH3+H2O����D��E2HOCH2COOH$��_{��}^{Ũ����}$
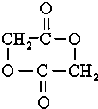 +2H2O��
+2H2O��
���� ��ϩֱ��������AΪCH3CHO����ȩ��һ�������õ�BΪCH3COOH�������Ϣ��֪����������������ȡ����Ӧ����CΪClCH2COOH��C����±����ˮ�⡢���ữ�õ�DΪHOCH2COOH��D����������Ӧ���ɻ���E���ݴ˽��
��� �⣺��ϩֱ��������AΪCH3CHO����ȩ��һ�������õ�BΪCH3COOH�������Ϣ��֪����������������ȡ����Ӧ����CΪClCH2COOH��C����±����ˮ�⡢���ữ�õ�DΪHOCH2COOH��D����������Ӧ���ɻ���E��
��1��������������֪��BΪCH3COOH��CΪ��ClCH2COOH���ʴ�Ϊ��CH3COOH��ClCH2COOH��
��2����A����������Ӧ����ʽΪ��CH3CHO+2Ag��NH3��OH$\stackrel{��}{��}$CH3COONH4+2Ag��+3NH3+H2O��
��D��E�ķ�Ӧ����ʽΪ��2HOCH2COOH$��_{��}^{Ũ����}$ +2H2O��
+2H2O��
�ʴ�Ϊ��CH3CHO+2Ag��NH3��OH$\stackrel{��}{��}$CH3COONH4+2Ag��+3NH3+H2O��
2HOCH2COOH$��_{��}^{Ũ����}$ +2H2O��
+2H2O��
���� ���⿼���л�����ƶ���ϳɣ��ѶȲ���ϸ������Ϣ�������ķ�Ӧ������˳�Ʒ������ƶϣ��������չ����ŵ�������ת���������ڻ���֪ʶ�Ĺ��̣�

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�| A�� | ���ԭ������������ | B�� | �˵���������� | ||
| C�� | ��������Ų��������Ա仯 | D�� | Ԫ�ػ��ϼ۳������Ա仯 |
| NaOH��Һ | ������Һ | ����Cu��OH��2 | ������ | |
| A | �����кͷ�Ӧ | - | Cu��OH��2�ܽ� | �������� |
| B | - | ������ | ����ש��ɫ���� | �������� |
| C | ����ˮ�ⷴӦ | ������ | ����ש��ɫ���� | - |
| D | ����ˮ�ⷴӦ | - | - | - |
A��CH3CH2COOH��
B��CH3CH��OH��CHO��
C��HCOOCH2CH3��
D��CH3COOCH3��
| A�� | ������ˮ����ֱ�����ͺ��ѻ����� | |
| B�� | ú��Һ�����������������仯 | |
| C�� | ú�к��б�����ͬϵ�����ú�ǹ�ҵ�ϻ�ñ�����Ҫ��Դ | |
| D�� | ʯ�ͷ����õ�ʯ�����У������顢���顢��ϩ����̬�� |
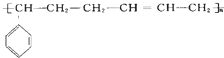 �ĸ߾���䵥��Ӧ�ǣ�������
�ĸ߾���䵥��Ӧ�ǣ��������ٱ���ϩ �ڶ�ϩ �۶���ϩ �ܱ�Ȳ �ݱ���ϩ��
| A�� | �� | B�� | �ܢ� | C�� | �٢� | D�� | �ۢ� |
| A�� | ԭ�Ӱ뾶��K��Na��S��O | B�� | ���Ӱ뾶��Na+��Mg2+��O2-��F- | ||
| C�� | ���ԣ�H2SO4��H3PO4��H2SiO3 | D�� | ���ԣ�KOH��Ca��OH��2��Mg��OH��2 |
| A�� | ����������ʴ���Ϊͬϵ�� | |
| B�� | �������Ͷ��ʴ�����Ϊͬ���칹�� | |
| C�� | 1 mol����������ʴ��Ļ������������������Ʒ�Ӧ����22.4 L���� | |
| D�� | ����������ʴ����ܷ���ȡ����Ӧ |
| A�� | Na2CO3��NaHCO3 | B�� | Na��Mg | C�� | KHCO3��K2CO3 | D�� | Si��Al |
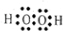 ��
��