��Ŀ����
����Ŀ��ijʵ��С���KSCN�����ʽ���̽�����������ʵ�飺
�Թ����Լ� | ʵ�� | �μ��Լ� | ���� |
KSCN��Һ | �� | i.�ȼ�1 mL 0.1 mol/L FeSO4��Һ ii.�ټ������ữ��KMnO4��Һ | i.���������� ii.�ȱ�죬����ɫ |
�� | iii.�ȵμ�1 mL 0.05 mol/L Fe2(SO4)3��Һ iv.�ٵμ�0.5 mL 0.5 mol/L FeSO4��Һ | iii.��Һ��� iv.��ɫ���Ա�dz |
(1)�������ӷ���ʽ��ʾʵ��I��Һ����ԭ��___________
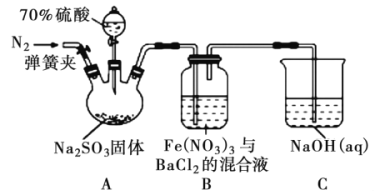
�����ʵ��I�к�ɫ��ȥ��ԭ��С��ͬѧ��Ϊ��SCN������KMnO4����ΪSO42���������ͼʵ��װ��֤ʵ�˲����dz����ġ�
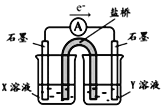
����X��Һ��_____________���������SO42�IJ�����������__________��
(2)���ʵ�������ɫ���Ա�dz����ʵ��С�����Ԥ�⡣
ԭ��٣�������ǿ����ʺ����������Ӽ�����ã�����֮��ǣ��������ǿ��������ЧӦ��������ЧӦ��ʹFe3++SCN![]() [Fe(SCN)]2+ƽ����ϵ�е�Fe3+��SCN��ϳ�[Fe(SCN)]2+�Ļ�����٣���Һ��ɫ��dz��
[Fe(SCN)]2+ƽ����ϵ�е�Fe3+��SCN��ϳ�[Fe(SCN)]2+�Ļ�����٣���Һ��ɫ��dz��
ԭ��ڣ�SCN������Fe2+��Ӧ������ɫ������ӣ���һ��ʹFe3++SCN![]() [Fe(SCN)]2+ƽ�����ƣ���ɫ���Ա�dz��
[Fe(SCN)]2+ƽ�����ƣ���ɫ���Ա�dz��
��֪��Mg2+��SCN����ϣ�����С�����������ʵ�飺
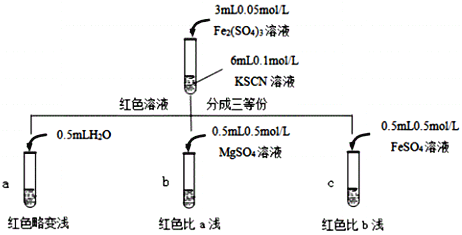
�ɴ��Ʋ⣬ʵ�������ɫ���Ա�dz����ԭ����___________________________��
���𰸡�MnO4+5Fe2++8H+==Mn2++5Fe3++4H2O��Fe3++3SCN![]() Fe(SCN)3 0.1 mol/L KSCN��Һ һ��ʱ���ȡ������Ӧ���KSCN��Һ���ȼ������ữ���ټ��Ȼ�����Һ�����ְ�ɫ���� ����ԭ���п���
Fe(SCN)3 0.1 mol/L KSCN��Һ һ��ʱ���ȡ������Ӧ���KSCN��Һ���ȼ������ữ���ټ��Ȼ�����Һ�����ְ�ɫ���� ����ԭ���п���
��������
��1����ʵ�����Һ��죬���������ӱ�������������йأ��������ӱ������������ӣ�
��SCN��������KMnO4����ΪSO42������Ƴ�ԭ��ط�Ӧ���ɵ���ת�Ʒ����֪���ʯīΪ������SCN����������X��ҺΪKSCN��Һ���ұ�ʯīΪ������Y��ҺΪKMnO4��Һ��������������ӣ��ɼ��������ữ���ټ����Ȼ������飻
��2��ʵ��ֱ����ˮ����Ũ�ȵ�����þ��������������Һ��ɫ���α�dz����˵��Ũ�ȡ���ЧӦ�Լ��������Ӷ�����ɫ��Ӱ�졣
��1����ʵ�����Һ��죬���������ӱ�������������йأ��������ӱ������������ӣ��漰��ӦΪMnO4��+5Fe2��+8H��=Mn2��+5Fe3��+4H2O��Fe3++3SCN![]() Fe(SCN)3 ��
Fe(SCN)3 ��
�ʴ�Ϊ��MnO4��+5Fe2��+8H��=Mn2��+5Fe3��+4H2O��Fe3++3SCN![]() Fe(SCN)3 ��
Fe(SCN)3 ��
��SCN��������KMnO4����ΪSO42������Ƴ�ԭ��ط�Ӧ���ɵ���ת�Ʒ����֪���ʯīΪ������SCN����������X��ҺΪKSCN��Һ���ұ�ʯīΪ������Y��ҺΪKMnO4��Һ��������������ӣ��ɼ��������ữ���ټ����Ȼ������飬������һ��ʱ���ȡ������Ӧ���KSCN��Һ���ȼ������ữ���ټ��Ȼ�����Һ�����ְ�ɫ������
�ʴ�Ϊ��0.1 mol��L��1 KSCN��Һ��һ��ʱ���ȡ������Ӧ���KSCN��Һ���ȼ������ữ���ټ��Ȼ�����Һ�����ְ�ɫ������
��2��ʵ��ֱ����ˮ����Ũ�ȵ�����þ��������������Һ��ɫ���α�dz���������Mg2+��SCN-����ϣ���˵��Ũ�ȡ���ЧӦ�Լ��������Ӷ�����ɫ��Ӱ�죬�ɽ���Ϊˮ��Һ��ϡ��ʹ��Һ��dz������ЧӦ��ʹFe3����SCN����ϳ�[Fe��SCN��] 2+�Ļ�����٣�SCN����Fe2����Ӧ������ɫ������ӣ����߿��ܾ��У�
�ʴ�Ϊ������ԭ���п��ܡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�������ݻ�ѧ֪ʶ�ش��������⣺
(1)ʵ����0.16g CH4��ȫȼ������224mLCO2(��״��)��0.36gҺ̬ˮ�����ų�8.903kJ����������CH4��ȼ����Ϊ________kJmol-1��
(2)̼�����Ƚ��ƶ������̷��������У�
i��MnCO3(s)![]() MnO(s)+CO2(g) H1=+a kJmol-1
MnO(s)+CO2(g) H1=+a kJmol-1
ii��2MnO (s)+ O2(g)![]() 2MnO2(s) H2=+b kJmol-1
2MnO2(s) H2=+b kJmol-1
��д������MnCO3��ȡMnO2���Ȼ�ѧ����ʽ��______________________________________��
(3)������ijͬѧ̽�����淴Ӧ2NO2(g)![]() N2O4(g) H=-56.9 kJmol-1�IJ���ʵ�鱨�棬�ݴ˻ش����⡣
N2O4(g) H=-56.9 kJmol-1�IJ���ʵ�鱨�棬�ݴ˻ش����⡣

���ձ���NO2��ĺ���ɫ��dz��˵��ƽ��2NO2(g)![]() N2O4(g)��________(�����Ӧ�����淴Ӧ��)�����ƶ�������NH4NO3��������ˮ��________(����ȡ������ȡ�)���̡�
N2O4(g)��________(�����Ӧ�����淴Ӧ��)�����ƶ�������NH4NO3��������ˮ��________(����ȡ������ȡ�)���̡�
(4)25��Cʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
��ѧʽ | CH3COOH | H2CO3 | HClO |
����ƽ�ⳣ�� | 1.7��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
�������ж�CH3COOH��H2CO3��HClO��������ǿ������˳����________________________
����Ŀ�����ⶨijHCl��Һ�����ʵ���Ũ�ȣ�����0.1000mol��L-1NaOH����Һ�����к͵ζ����÷�̪��ָʾ������
��ش��������⣺

(1)����ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ1.10mL���ζ���Һ����ͼ�����ʱ���ı���Һ�����Ϊ ___________��
(2)��ѧ����������ƽ��ʵ�飬���ݼ�¼���£�
ʵ�� ��� | ����HCl��Һ�����/mL | 0.1000mol��L-1NaOH��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 25.00 | 0.00 | 26.11 |
2 | 25.00 | 1.56 | 31.30 |
3 | 25.00 | 0.22 | 26.31 |
ѡȡ�����������ݣ������HCl������Һ�����ʵ���Ũ��Ϊ ________mol��L-1��С���������λ����
(3)�ζ�ʱ����ȷ������_____________________________________________________���ζ��ﵽ�յ��������_____________________________________________����ʱ��ƿ����Һ��pH�ķ�Χ��________��
(4)������Щ������ʹ�ⶨ���ƫ��_______________������ţ���
A����ƿ������ˮϴ�������ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ���