��Ŀ����
10��������ϩ�����Ʊ�����ϩ����ԭ�ϣ���;�㷺��ij���Ʊ�������ϩ�����������£�
��1��������ϩ���й����ŵ����ƣ�������̼̼˫������Ӧ���ͣ��ټӳɷ�Ӧ����ȡ����Ӧ����ˮ�ⷴӦ����
��2��HO-CH2-CHO�����ƣ�ϵͳ��������2-�ǻ���ȩ��
��3��д����Ӧ�۵ķ���ʽ��2CH3CHO+O2 $��_{��}^{����}$2CH3COOH��
��4��д����������Ҫ��Ĵ�����ϩ����ͬ���칹��Ľṹ��ʽ��
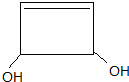 ��
���ٷ����к��л��Ľṹ
��1mol�������������Ľ����Ʒ�Ӧ����1mol���壮
�ۺ˴Ź�������ֻ��ʾ3�����շ��ҷ�ĸ߶���ͬ
��5������˵������ȷ����acd
a��ʵ������ȡ��ϩ��ȡˮԡ����
b�����������������백����Ӧ������
c����Ȳ�к��е���ϩ���ʿ���ͨ�����Ը�����س�ȥ
d��A��B�����Цм���
���� �����������ͼ˳�ƣ��Ʊ�������ϩ��������Ϊ��CH2�TCH2��98%�����ᷴӦ����������������
��Ӧ��Ϊ��CH2�TCH2+HO-SO2OH��Ũ��$\stackrel{һ������}{��}$CH3CH2OSO2OH��
����������ˮ�⣬��Ӧ��Ϊ��CH3CH2OSO2OH+H2O$\stackrel{90��}{��}$CH3CH2OH+HO-SO2OH
�Ҵ�������������ȩ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O
��ȩ�����������ᣬ��Ӧ��Ϊ��2CH3CHO+O2 $��_{��}^{����}$2CH3COOH
�������Ȳ��Ӧ���ɴ�����ϩ��CH3COOH+CH��CH$\stackrel{һ������}{��}$CH3COOCH=CH2
��1�����ݴ�����ϩ���Ľṹ��ʽ��������еĹ����ţ����ݷ�Ӧ���٢��ж��䷴Ӧ���ͣ�
��2��HO-CH2-CHO�����ǻ���ȩ�����൱����ȩ�е��ⱻ�ǻ�ȡ�����ݴ�ϵͳ������
��3����Ӧ��Ϊ��ȩ�����������ᣬ�ݴ���д����ʽ��
��4��������ϩ��ΪCH3COOCH=CH2��ͬ���칹�壬�����к��л��Ľṹһ�����൱��1��̼̼˫����1mol�������������Ľ����Ʒ�Ӧ����1mol����˵������2mol�ǻ����˴Ź�������ֻ��ʾ3�����շ��ҷ�ĸ߶���ͬ��˵������3���⣬����ԭ�Ӹ�������ͬ��
��5��a��ˮԡ���ȵ��¶�1-100�ȣ�
b�������������백���������ⷴӦ�����Σ�
c����Ȳ�к��е���ϩ��������ͨ�����Ը��������Һ�У���Ȳ��ϩ���������ɶ�����̼���壻
d��̼̼˫����̼��˫�����Цм�������Ϊ�ң�A��B�ֱ�Ϊ�Ҵ������ᣬ�Ҵ������м���
��� �⣺�����������ͼ˳�ƣ��Ʊ�������ϩ��������Ϊ��CH2�TCH2��98%�����ᷴӦ����������������
��Ӧ��Ϊ��CH2�TCH2+HO-SO2OH��Ũ��$\stackrel{һ������}{��}$CH3CH2OSO2OH��
����������ˮ�⣬��Ӧ��Ϊ��CH3CH2OSO2OH+H2O$\stackrel{90��}{��}$CH3CH2OH+HO-SO2OH
�Ҵ�������������ȩ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O
��ȩ�����������ᣬ��Ӧ��Ϊ��2CH3CHO+O2 $��_{��}^{����}$2CH3COOH
�������Ȳ��Ӧ���ɴ�����ϩ��CH3COOH+CH��CH$\stackrel{һ������}{��}$CH3COOCH=CH2
��1���������Ȳ��Ӧ���ɴ�����ϩ��CH3COOH+CH��CH$\stackrel{һ������}{��}$CH3COOCH=CH2��������ϩ��ΪCH3COOCH=CH2�����еĹ�����Ϊ������̼̼˫����
��Ӧ��Ϊ��CH2�TCH2+HO-SO2OH��Ũ��$\stackrel{һ������}{��}$CH3CH2OSO2OH��Ϊϩ���ļӳɷ�Ӧ��
����������ˮ�⣬��Ӧ��Ϊ��CH3CH2OSO2OH+H2O$\stackrel{90��}{��}$CH3CH2OH+HO-SO2OH��Ϊ����ˮ������ȡ����Ӧ��
�ʴ�Ϊ��������̼̼˫�����ӳɷ�Ӧ��ȡ����Ӧ��
��2��HO-CH2-CHO�����ǻ���ȩ�������л���̼������2��C������Ϊ��ȩ�����ڵ�̼��Ϊ2��λ�����ǻ���
�������ƣ�ϵͳ��������2-�ǻ���ȩ��
�ʴ�Ϊ��2-�ǻ���ȩ��
��3����Ӧ��Ϊ��ȩ�����������2CH3CHO+O2 $��_{��}^{����}$2CH3COOH��
�ʴ�Ϊ��2CH3CHO+O2 $��_{��}^{����}$2CH3COOH��
��4��������ϩ��ΪCH3COOCH=CH2��ͬ���칹�壬�����к��л��Ľṹһ�����൱��1��̼̼˫����1mol�������������Ľ����Ʒ�Ӧ����1mol����˵������2mol�ǻ����˴Ź�������ֻ��ʾ3�����շ��ҷ�ĸ߶���ͬ��˵������3���⣬����ԭ�Ӹ�������ͬ�����Է���������ͬ���칹��Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��5��a��ʵ������ȡ��ϩ�¶�Ϊ170�ȣ�����ˮԡ�ﵽ���¶ȣ���a����
b�����������������백������������ˮ��İ��ⷴӦ�����Σ���b��ȷ��
c����Ȳ�к���C��C�����ţ���ϩ�к���C=C�����ţ����ܱ����Ը��������Һ���������ɶ�����̼���壬��c����
d��A��B�ֱ�Ϊ�Ҵ�CH3CH2OH������CH3COOH�������к���̼��˫�������Цм����Ҵ���������d����
��ѡacd��
���� ���⿼���л���ĺϳɣ���Ŀ�Ѷ��еȣ������ע���������Ϣ�����ŵ�ת�����ر����л�������ŵ����ʣ��ǽ�����Ĺؼ����״���Ϊ��5��ע�����֪ʶ�����գ�

 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�| A�� | x=10ʱ����Һ����NH4+��Al3+��SO42-����c��NH4+��=c��Al3+�� | |
| B�� | x=20ʱ����Һ���������ķ�Ӧ���ӷ���ʽΪ��Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+AlO2-+2H2O | |
| C�� | x=30ʱ����Һ����Ba2+��AlO2-��OH-����c��OH-����c��AlO2-�� | |
| D�� | �μӹ����У����ɳ�����������ʵ���Ϊ0.003mol |
| A�� | 3mol/L��ˮ | B�� | 4mol/L HNO3 | C�� | 8mol/L NaOH | D�� | 18mol/L H2SO4 |
| A�� | ����NaOH��Һ���а�ɫ����������˵��������һ����Mg2+ | |
| B�� | ����AgNO3��Һ���а�ɫ����������˵��������һ����Cl- | |
| C�� | ���������ữ��BaCl2��Һ���а�ɫ����������˵��������һ����SO42- | |
| D�� | ����ŨNaOH��Һ�ȣ��Թܿڵ�ʪ���ɫʯ����ֽ������˵��������һ����NH4+ |
| A�� | ������Һ�п��ܴ������ڣ�Ca2+��Cl-��SO42-��K+ | |
| B�� | ��������Һ�п��ܴ������ڣ�Cu2+��NH4+��NO3-��Cl- | |
| C�� | ʹʯ����Һ��������Һ�п��ܴ������ڣ�Na+��Al3+��SO42-��NO3- | |
| D�� | ˮ�����c��OH-��=10-10mol/L��Һ�п��ܴ������ڣ�l-��NO3-��Mg2+��K+ |
| A�� | ���Ͻ��۵�ͣ������������Ŵ� | |
| B�� | �������Ⱦ���ǿ�����ԣ�������Ư��֯�� | |
| C�� | �������ƹ���ʵ���ɫ���������������� | |
| D�� | ����ͭ����ˮ�����ԣ���������ɱ���� |
| A�� | ��2mol��Cl2ͨ�뵽��1mol��FeBr2����Һ�У�2Fe2++2Br-+2Cl2�T2Fe3++4Cl-+Br2��ȷ��Cl2������Fe2+��Br-�������� | |
| B�� | ��Cu�缫���NaCl��Һ�����ĵ缫��Ӧʽ��2Cl--2e-�TCl2����ȷ��Cl-������OH-�ŵ� | |
| C�� | ����SO2ͨ�뵽NaClO��Һ�У�SO2+H2O+ClO-�THClO+HSO3-��ȷ��H2SO3������ǿ��HClO | |
| D�� | Mg��HCO3��2��Һ��������NaOH��Һ��Ӧ��Mg2++2HCO3-+4OH-�TMg��OH��2��+2CO32-+2H2O��ȷ��Mg��OH��2��MgCO3������ |
| A�� | ��Ӧ�����Ӱ뾶�Ĵ�С˳��Ϊ��D��B��A | |
| B�� | C������������Ӧˮ��������Ա�D��ǿ | |
| C�� | A�ֱ���B��C�γɵĻ������л�ѧ��������ͬ | |
| D�� | B��C���ʾ��ܺ�����������Һ������Ӧ�������� |