��Ŀ����
����Ŀ����1�������Ȼ�����Һ�ش��������⣺
����FeCl3��Һ�м�������NaHCO3������������Ϊ___�������ӷ���ʽ��ʾ��ԭ��___��
�ڲ��ϼ���FeCl3��Һ������ˮ�ֲ����գ��õ��Ĺ�����___��
��������FeCl3��Һʱ��Ϊ��ֹ��Һ����ǣ�Ӧ����___��
��2�����÷�ӦCu+H2O2+H2SO4=CuSO4+2H2O���һ��ԭ��أ��ش��������⣺
�ٸ�������Ϊ___��������ӦʽΪ___��
�ڷ�Ӧ������SO![]() ��____���ƶ���
��____���ƶ���
�۵���·��ת��0.1mol����ʱ�����Һ����(�����缫)������___�ˡ�
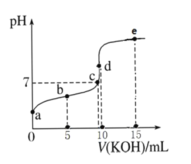
��3����֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ���ش��������⣺
��ѧʽ | CH3COOH | H2CO3 | HClO |
����ƽ�ⳣ�� | Ka=1.8��10-5 | Ka1=4.3��10-7 Ka2=5.6��10-11 | Ka=3.0��10-8 |
�����ʵ���Ũ�Ⱦ�Ϊ0.1molL-1��������Һ��pH��С�������е�˳����__���ñ����д��
a.CH3COONa b.Na2CO3 c.NaClO d.NaHCO3
�ڳ����£�0.1molL-1CH3COOH��Һ��ˮϡ�����У����б���ʽ�����ݱ�����___������ĸ��
A.c��H+�� B.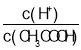 C.c��H+��c��OH-�� D.
C.c��H+��c��OH-�� D.![]() E.
E.![]()
��д�������������Һ��ͨ������������̼�����ӷ���ʽ��____��
��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ������û��ҺpH=6������Һ��c��CH3COO-��-c��Na+��=____����ȷ��ֵ����
�ݱ�״���£���1.12LCO2ͨ��100mL1molL-1��NaOH��Һ�У�����Һ������Ũ�ȷ���������е�ʽ��c��OH-��=2c��H2CO3��+____��
���𰸡�������ͺ��ɫ�������� Fe3++3HCO![]() =3CO2��+Fe(OH)3�� Fe2O3 Ũ���� Cu H2O2+2H++2e-=2H2O �� 3.2 a��d��c��b BD ClO-��H2O��CO2=HCO
=3CO2��+Fe(OH)3�� Fe2O3 Ũ���� Cu H2O2+2H++2e-=2H2O �� 3.2 a��d��c��b BD ClO-��H2O��CO2=HCO![]() ��HClO 9.9��10-7molL-1 c��HCO
��HClO 9.9��10-7molL-1 c��HCO![]() ��+c��H+��
��+c��H+��
��������
��1������FeCl3��Һ�м�������NaHCO3������˫ˮ�⣬�������������Ͷ�����̼���壬����������Ϊ������ͺ��ɫ���������������ӷ���ʽ��ʾ��ԭ��Fe3++3HCO![]() =3CO2��+Fe(OH)3�����ʴ�Ϊ��������ͺ��ɫ����������Fe3++3HCO
=3CO2��+Fe(OH)3�����ʴ�Ϊ��������ͺ��ɫ����������Fe3++3HCO![]() =3CO2��+Fe(OH)3����
=3CO2��+Fe(OH)3����
�ڲ��ϼ���FeCl3��Һ������ˮ�ֲ����գ���������FeCl3��Һʱ��FeCl3ˮ����������������HCl�����ȴٽ�HCl�ӷ����Ӷ��ٽ�FeCl3ˮ�⣬����ʱ�õ������������壬���������������壬���������ֽ�������������ˮ���õ��Ĺ�����Fe2O3���ʴ�Ϊ��Fe2O3��
��FeCl3ˮ����������������HCl��Ϊ�����Ȼ���ˮ�⣬����Һ�еμ�Ũ���ἴ�ɡ��ʴ�Ϊ��Ũ���
��2�����÷�ӦCu+H2O2+H2SO4=CuSO4+2H2O���һ��ԭ��أ��ٸ÷�Ӧ��CuԪ�ػ��ϼ���0�۱�Ϊ+2�ۡ�OԪ�ػ��ϼ���-1�۱�Ϊ-2�ۣ�Ҫ���÷�Ӧ��Ƴ�ԭ��أ�Cu��������������H2O2�õ��ӷ�����ԭ��Ӧ����Ϊ�����������£�����H2O2�õ��Ӻ������ӷ�Ӧ����ˮ���缫��ӦʽΪH2O2+2H++2e-=2H2O��������������ΪCu��������ӦʽΪH2O2+2H++2e-=2H2O���ʴ�Ϊ��Cu��H2O2+2H++2e-=2H2O��
��ԭ����У����������ƶ�����Ӧ������SO![]() ���ƶ����ʴ�Ϊ������
���ƶ����ʴ�Ϊ������
�۵���·��ת��0.1mol����ʱ������Cu��2e��=Cu2��������0.05molCu2�������Һ����(�����缫)������0.05mol��64g��mol��1=3.2�ˡ��ʴ�Ϊ��3.2��
��3����Na2CO3��ǿ�������Σ�̼�������ˮ�����Һ�ʼ��ԣ�NaClO��ǿ��������ˮ���Լ��ԣ�CH3COONa��ǿ�������Σ����������ˮ�����Һ�ʼ��ԣ�NaHCO3��ǿ�������Σ�ˮ���Լ��ԣ�������CH3COOH>H2CO3>HClO>HCO![]() �����������Խ���������ˮ������Խǿ����ˮ��������CH3COO��<HCO
�����������Խ���������ˮ������Խǿ����ˮ��������CH3COO��<HCO![]() <ClO��<CO
<ClO��<CO![]() ������Һ�ļ���Na2CO3>NaClO>NaHCO3>CH3COONa����pH��a��d��c��b���ʴ�Ϊ��a��d��c��b��
������Һ�ļ���Na2CO3>NaClO>NaHCO3>CH3COONa����pH��a��d��c��b���ʴ�Ϊ��a��d��c��b��
��A��CH3COOH��Һ��ˮϡ���̣���ٽ����룬��c��H������С����A��ѡ��
B��![]() ��CH3COOH��Һ��ˮϡ���̣��ٽ����룬���������ʵ������������ʵ�����С����ϡ�����б�ֵ���Bѡ��
��CH3COOH��Һ��ˮϡ���̣��ٽ����룬���������ʵ������������ʵ�����С����ϡ�����б�ֵ���Bѡ��
C��ϡ���̣�c��H������С��c��OH��������c��H������c��OH����=Kw��Kwֻ���¶�Ӱ�����Բ��䣬��C��ѡ��
D��ϡ���̣�c��H������С��c��OH����������![]() ���Dѡ��
���Dѡ��
E.![]() �Ǵ���ĵ��볣����ֻ���¶��йأ��¶Ȳ��䣬
�Ǵ���ĵ��볣����ֻ���¶��йأ��¶Ȳ��䣬![]() ���䣬��E��ѡ��
���䣬��E��ѡ��
�ʴ�Ϊ��BD��
������H2CO3>HClO>HCO3���������������Һ��ͨ������������̼����̼�����ƺʹ����ᣬ���ӷ���ʽ��ClO-��H2O��CO2=HCO![]() ��HClO���ʴ�Ϊ��ClO-��H2O��CO2=HCO
��HClO���ʴ�Ϊ��ClO-��H2O��CO2=HCO![]() ��HClO��
��HClO��
��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ�����ڵ���غ㣺c��Na����+c��H����=c��OH����+c��CH3COO����������û��ҺpH=6������Һ��c��CH3COO-��-c��Na+��=c��H����-c��OH����=10-6mol��L��1-10-8mol��L��1=9.9��10-7mol��L��1���ʴ�Ϊ��9.9��10-7molL-1��
�ݱ�״���£�1.12L CO2�����ʵ���Ϊ��![]() =0.05mol���������Ƶ����ʵ���Ϊ��1mol��L-1��0.1L=0.1mol������ǡ����ȫ��Ӧ����̼���ƣ�����̼������Һ�е������غ�ɵã�c��OH-��=2c��H2CO3��+c��HCO
=0.05mol���������Ƶ����ʵ���Ϊ��1mol��L-1��0.1L=0.1mol������ǡ����ȫ��Ӧ����̼���ƣ�����̼������Һ�е������غ�ɵã�c��OH-��=2c��H2CO3��+c��HCO![]() ��+c��H+�����ʴ�Ϊ��c��HCO
��+c��H+�����ʴ�Ϊ��c��HCO![]() ��+c��H+����
��+c��H+����

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�