��Ŀ����
11��������������Ni2O3����һ����Ҫ�ĵ���Ԫ�����Ϻ����ز��ϣ���ҵ�����ú������ϣ����������ơ�þ�Ͻ�Ϊ������ȡ��������NiC2O4•2H2O�����ٸ������ղ�������ȡ��������������֪����ĸơ�þ�����ξ�������ˮ���������й�������ʾ��ͼ�ش����⣮
��1������1Ϊ�����ܽ⣬���ˣ�
��2�����������ж�ν��й��ˣ�ʵ���ҽ��й��˲��������õ�������������ʵ������в�������������ȫ��ͬ����BC����ѡ����ĸ����
������0.1mol/L��H2SO4��Һ �ڲⶨNa2CO3��Һ��pH
����KI������ֽ������Һ�е����������� �ܼ���ʳ����Һ�Ʊ�NaCl����
������20%��KNO3��Һ
A���٢�B���ڢ�C���ܢ�D���٢�
��3������H2O2��������Ҫ��Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+�T2Fe3++2H2O��
����Na2CO3��Һ��pH��4.0��4.5����Ŀ��Ϊ�ٽ�Fe3+ˮ�������ȫ������NH4F�������������Ca2+��Mg2+��
��4����������NiC2O4•2H2O�����ȿ����и�����ˮ���ڸ�����������Сʱ���Ƶ�Ni2O3��ͬʱ��û�����壮���������ȷֽ�Ļ�ѧ����ʽΪ2NiC2O4$\frac{\underline{\;����\;}}{\;}$Ni2O3+3CO��+CO2����
��5����ҵ�ϻ����õ�ⷨ��ȡNi2O3����NaOH��Һ����NiCl2��Һ��pH��7.5����������Na2SO4����ö��Ե缫��⣮�������в�����Cl2��80%������������������ClO-���ٰѶ���������Ϊ��������ClO-����Ni��OH��2����Ni2O3�����ӷ���ʽΪClO-+2Ni��OH��2�TCl-+Ni2O3+2H2O��
���� ���̷����������ϣ����������ơ�þ�Ͻ�Ϊ������ȡ��������NiC2O4•2H2O�����ٸ������ղ�������ȡ�����������������ϼ������ܽ����˵õ���Һ�к����������ӡ������ӡ�þ���ӡ������ӣ������������������������Ϊ�����ӣ�����̼������Һ������Һ��PH��ʹ������ȫ�����������˺����Һ�ڼ���NH4F���������Ӻ�þ���ӣ����˵õ���Һ���������ӵ���Һ������Һ�м���������ɲ�����������Ũ���ᾧ��������ΪĿ�����NiC2O4•2H2O��
��1������������Ҫ�����������ơ�þ�Ͻ�Ϊ���������������ܺ���ˣ��ܽ��������ȥ�����
��2��������O.1mol/L��H2SO4��Һ�������������ã�
�ڲⶨNa2CO3��Һ��pH��������պȡ��Һ����pH��ֽ�ϲⶨ��
����KI������ֽ������Һ�����������ӣ�������պȡ��Һ����KI������ֽ�ϲⶨ��
�ܼ���ʳ����Һ�Ʊ�NaCl���壬�������������ֲ����ȹ���ɽ���
������20%��KNO3��Һ����������������ܽ⣻
��3����������������������Ϊ�����ӣ����ݵ���غ��ԭ���غ㣬����Na2CO3��Һ��pH��4.0〜4.5���ٽ�������ˮ���γ��������������������ơ�����þ������ˮ��
��4����������NiC2O4•2H2O�����ȿ����и�����ˮ������NiC2O4��NiC2O4�ٷ���������ԭ��Ӧ��Ni��+2�����ߵ�+3�ۣ���C��+3�۽��͵�+2�ۣ�����Ҫ�����ɻ�����壬����һ����ΪCO2������Ni2O3��CO��CO2�������û��ϼ����������ƽ��
��5��Cl��+1�۽��͵�-1�ۣ�Ni��+2�����ߵ�+3�ۣ����û��ϼ�������ȿ���ƽClO-��Ni��OH��2��Cl-��Ni2O3��ϵ����������Hԭ���غ���ƽˮ��ϵ������������Oԭ�Ӽ����ƽ�Ƿ���ȷ�����ݻ�ѧ����ʽ��Ԫ���غ����õ���
��� �⣺���̷����������ϣ����������ơ�þ�Ͻ�Ϊ������ȡ��������NiC2O4•2H2O�����ٸ������ղ�������ȡ�����������������ϼ������ܽ����˵õ���Һ�к����������ӡ������ӡ�þ���ӡ������ӣ������������������������Ϊ�����ӣ�����̼������Һ������Һ��PH��ʹ������ȫ�����������˺����Һ�ڼ���NH4F���������Ӻ�þ���ӣ����˵õ���Һ���������ӵ���Һ������Һ�м���������ɲ�����������Ũ���ᾧ��������ΪĿ�����NiC2O4•2H2O��
��1��������ͼ�������ܡ����ɺ����������ơ�þ���ӵ���Һ�������ܽ⣬�ܽ��������ȥ��������ˣ�
�ʴ�Ϊ�������ܽ⣬���ˣ�
��2��������O.1mol/L��H2SO4��Һ�������������ã�
�ڲⶨNa2CO3��Һ��pH��������պȡ��Һ����pH��ֽ�ϲⶨ��
����KI������ֽ���� ��Һ�����������ӣ�������պȡ��Һ����KI������ֽ�ϲⶨ��
�ܼ���ʳ����Һ�Ʊ�NaCl���壬�������������ֲ����ȹ���ɽ���
������20%��KNO3��Һ����������������ܽ⣻
���Ԣڢۺ͢ܢݷ��ϣ�
�ʴ�Ϊ��BC��
��3����˫��ˮ��Ŀ������������Fe3+����Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+�T2Fe3++2H2O������̼������Һ�������ǵ���pH���ٽ�������ˮ�������ȫ���ټ���NH4F��Ŀ���dz�ȥ�����ӡ�þ���ӣ������Ӳ���ʱ���ɲ�����������
�ʴ�Ϊ��2Fe2++H2O2+2H+�T2Fe3++2H2O���ٽ�������ˮ�������ȫ��Ca2+��Mg2+��
��4����������NiC2O4•2H2O�����ȿ����и�����ˮ������NiC2O4��NiC2O4�ٷ���������ԭ��Ӧ��Ni��+2�����ߵ�+3�ۣ���C��+3�۽��͵�+2�ۣ�����Ҫ�����ɻ�����壬����һ����ΪCO2������Ni2O3��CO��CO2�������û��ϼ�������ȣ�Ni������2����3-2����C������1����4-3����C�����ͣ�3����3-2������ƽ����ʽΪ��2NiC2O4$\frac{\underline{\;����\;}}{\;}$Ni2O3+3CO��+CO2����
�ʴ�Ϊ��2NiC2O4$\frac{\underline{\;����\;}}{\;}$Ni2O3+3CO��+CO2����
��5��Cl��+1�۽��͵�-1�ۣ�Ni��+2�����ߵ�+3�ۣ����û��ϼ�������ȿ���ƽClO-��Ni��OH��2��Cl-��Ni2O3��ϵ����������Hԭ���غ���ƽˮ��ϵ������������Oԭ�Ӽ����ƽ�Ƿ���ȷ���õ����ӷ���ʽΪ��ClO-+2Ni��OH��2�TCl-+Ni2O3+2H2O��
�ʴ�Ϊ��ClO-+2Ni��OH��2�TCl-+Ni2O3+2H2O��
���� ���⿼�������̷����ƶϣ�ʵ���������ķ����жϣ���ѧ����ʽ����͵��ԭ������Ӧ�ã���Ŀ�ۺ��Խϴ��ѶȽϴ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��NaCl��NaBr��NaI�����Һ��ͨ��F2��I-��Br-��Cl- | |
| B�� | ��NaCl��NaI��Na2S�����Һ�еμ�AgNO3��Һ��S2-��I-��Cl- | |
| C�� | ��FeCl3��CuCl2��HCl�����Һ�м���Zn�ۣ�Cu2+��Fe3+��H+ | |
| D�� | ��NaAlO2��Na2CO2��NaOH�����Һ�еμ�ϡ���AlO2-��CO32-��HCO3-��OH- |
| A�� | NaHSO4�����ڣ��TNa++H++SO42- | B�� | NaHCO3�TNa++H++CO32- | ||
| C�� | CH3COOH?CH3COO-+H+ | D�� | H2CO3?2H++CO32- |

| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 7.6 | 9.7 |
| Fe3+ | 2.7 | 3.7 |
| Al3+ | 3.8 | 4.7 |
| Mn2+ | 8.3 | 9.8 |
�����������̣��ش��������⣺
��1��д�������������������Ӧ�Ļ�ѧ����ʽ��SO2+MnO2�TMnSO4��
��2������Һ��pH��2�����������̿������ܵ�ԭ��Ϊ������������ˮ������Ӧ��SO2+H2O=H2SO3�����ɵ�H2SO3���ֵ��룺H2SO3?H++HSO3-���û�ѧ����ͱ�Ҫ������˵������
��3�������ӷ���ʽ��ʾ����������̵����ã�MnO2+2Fe2++4H+�TMn2++2Fe3++2H2O���ӱ��������������ܲ���ȡ��������������̡��IJ��裿ԭ���Dz��ܣ�pH=9.7ʱFe2+����ȫ��������pH=8.3ʱMn2+�Ѿ���ʼ������
�����Լ�������������̵���A������ţ���
A��˫��ˮ B����ˮ C�����������Һ D����������
��4����ͬѧ��Ϊ������̼���̣�MnCO3������������[Mn��OH��2]���ʯ���飬���Ƿ�ͬ��˹۵㣿�������ɣ�ͬ�⣬�����ӡ�������ˮ��̶ȱ������Ӵ���̼���̻����������������ӷ�Ӧ���ٽ������ӡ�������ˮ�⣬����ת��Ϊ��������ͨ�����˳�ȥ��
��5���Ӻ������̵���Һ����ȡ�����̾���IJ���������Ũ�������½ᾧ�����ˣ�������������ȡ�ߴ��ȵ����죬������������̣���������������������������Һ�У��ٽ��й��ˡ�ϴ�ӡ����������տɵ���������
| A�� | ������C��H��O�ĸ�����Ϊ1��2��3 | B�� | ������C��H�ĸ�����Ϊ1��2 | ||
| C�� | ���л������Է�������Ϊ14 | D�� | �÷����п϶������� |
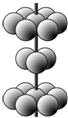 X��Y��Z��MΪǰ�������г�������ԭ�������������������Ԫ�أ�X��̬ԭ��δ�ɶԵ�������������������ࣻYԪ��ԭ�Ӻ����3���ܼ���������ܼ���������ǰ�����ܼ�������֮�ͣ�Z�ĵ��ʳ�����Ϊ����ɫ���壬ZY3���ӳ�ƽ���������Σ�Mԭ����Χ�����Ų�ʽΪ3dn4sn��
X��Y��Z��MΪǰ�������г�������ԭ�������������������Ԫ�أ�X��̬ԭ��δ�ɶԵ�������������������ࣻYԪ��ԭ�Ӻ����3���ܼ���������ܼ���������ǰ�����ܼ�������֮�ͣ�Z�ĵ��ʳ�����Ϊ����ɫ���壬ZY3���ӳ�ƽ���������Σ�Mԭ����Χ�����Ų�ʽΪ3dn4sn��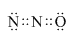 ��
��