��Ŀ����
11��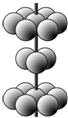 X��Y��Z��MΪǰ�������г�������ԭ�������������������Ԫ�أ�X��̬ԭ��δ�ɶԵ�������������������ࣻYԪ��ԭ�Ӻ����3���ܼ���������ܼ���������ǰ�����ܼ�������֮�ͣ�Z�ĵ��ʳ�����Ϊ����ɫ���壬ZY3���ӳ�ƽ���������Σ�Mԭ����Χ�����Ų�ʽΪ3dn4sn��
X��Y��Z��MΪǰ�������г�������ԭ�������������������Ԫ�أ�X��̬ԭ��δ�ɶԵ�������������������ࣻYԪ��ԭ�Ӻ����3���ܼ���������ܼ���������ǰ�����ܼ�������֮�ͣ�Z�ĵ��ʳ�����Ϊ����ɫ���壬ZY3���ӳ�ƽ���������Σ�Mԭ����Χ�����Ų�ʽΪ3dn4sn����ش��������⣺
��1��X��Y��Z����Ԫ��ԭ�ӵ�һ�������ɴ�С��˳����N��O��S������Ԫ�ط��ű�ʾ��
��2��ijX�⻯����ӽṹʽΪ��H-X=X-H���÷�����Xԭ�ӵ��ӻ���ʽ��sp2��YԪ�ؼ��⻯��ķе����Z���⻯���Ҫԭ����H2O���Ӽ��γ����������H2S���Ӽ䲻���γ������
��3�����ݵȵ���ԭ����д��X2Y���ӵĵ���ʽ��
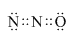 ��
����4��M�����ԭ�Ӷѻ���ʽΪ�����ѻ�������ͼ��ʾ����������Mԭ����λ����12��ijM����ﻯѧʽ��[M��H2O��5Cl]Cl2•H2O��1mol��������к���λ������Ŀ��6mol��
���� YԪ��ԭ�Ӻ����3���ܼ�����Ϊ1s��2s��2p3���ܼ���������ܼ���������ǰ�����ܼ�������֮�ͣ���2p�ܼ�����4�����ӣ�Y�ĺ�������Ų�ʽΪ��1s22s22p4��ΪOԪ�أ�
X��̬ԭ��δ�ɶԵ�������������������࣬��X���ڢ�A�壬���ԭ��������֪XΪNԪ�أ�
Z�ĵ��ʳ�����Ϊ����ɫ���壬��ZΪSԪ�أ�
M��ԭ����Χ�����Ų�ʽΪ3dn4sn����n=2����M��̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d24s2����ԭ������Ϊ22����MΪTiԪ�أ��ݴ˽��н��
��� �⣺YԪ��ԭ�Ӻ����3���ܼ�����Ϊ1s��2s��2p3���ܼ���������ܼ���������ǰ�����ܼ�������֮�ͣ���2p�ܼ�����4�����ӣ�Y�ĺ�������Ų�ʽΪ��1s22s22p4��ΪOԪ�أ�X��̬ԭ��δ�ɶԵ�������������������࣬��X���ڢ�A�壬���ԭ��������֪XΪNԪ�أ�Z�ĵ��ʳ�����Ϊ����ɫ���壬��ZΪSԪ�أ�M��ԭ����Χ�����Ų�ʽΪ3dn4sn����n=2����M��̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d24s2����ԭ������Ϊ22����MΪTiԪ�أ�
��1���ǽ�����Խǿ����һ������Խǿ������NԪ�ص�2p������ڰ���״̬�����һ�����ܴ���OԪ�أ��������ߵĵ�һ�����ܴ�СΪ��N��O��S��
�ʴ�Ϊ��N��O��S��
��2��XΪNԪ�أ�����˫���к���1���Ҽ���H-N=N-H�л�����1����������������1�Թµ��Ӷԣ�����Nԭ���ܹ�����3���ӻ������Ϊsp2�ӻ�������H2O���Ӽ��γ����������H2S���Ӽ䲻���γ����������ˮ�ķе�������⣬
�ʴ�Ϊ��sp2��H2O���Ӽ��γ����������H2S���Ӽ䲻���γ������
��3��X2YΪN2O��������к���22�����ӣ��������̼��Ϊ�ȵ����壬��ṹ�������̼���ƣ���������˫���������ʽΪ��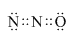 ��
��
�ʴ�Ϊ��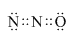 ��
��
��4����ͼ��֪�������ѻ���ʽΪþ�ͣ������������ѻ����Զ����ԭ�ӷ�����λ�����ĵ�ԭ����֮���ڣ�1������ԭ��Ϊ12���湲�ã�����λ��Ϊ12��
[Ti��H2O��5Cl]Cl2•H2O�У�1molTi��1molCl��5molH2O�γ���6mol��λ����
�ʴ�Ϊ��12�� 6 mol����6��6.02��1023����
���� ���⿼����λ�á��ṹ�����ʹ�ϵ��Ӧ�ã���Ŀ�Ѷ��еȣ��漰��һ�����ܴ�С�Ƚϡ��ȵ������Ӧ�á������ṹ������������֪ʶ����ȷ�ƶϸ�Ԫ������Ϊ���ؼ���������ؿ���ѧ���ķ���������������

��ȡ������Һ����������NaOHŨ��Һ�����յõ����ɫ���������������ݲ��������˺����Һ����εμ�ϡ����������������������������
����ȡ����ԭ��Һ������ϡ������Һ����������������ڿ����б�Ϊ��ɫ��
��������ʵ����ʵ�ƶϣ��йظ���Һ��˵��������ǣ�������
| A�� | ���ٺ���4������ | |
| B�� | ȡ����ԭ��Һ�ȼ������ᣬ�����BaCl2��Һ���϶��������ɫ���� | |
| C�� | ��ԭ��Һ�м��������ữ����������Һ������ɫ����������ƶ���Һ�к���K+ | |
| D�� | ��Һ�п��ܺ���Fe3+��NH4+��SO42-��Cl-��NO3- |
 ��˹����Aspartame���ṹ��ʽ��ͼ����������ˬ����ζ�����ԼΪ���ǵ�200���������йذ�˹����˵������ȷ���ǣ�������
��˹����Aspartame���ṹ��ʽ��ͼ����������ˬ����ζ�����ԼΪ���ǵ�200���������йذ�˹����˵������ȷ���ǣ�������| A�� | ����ʽΪC14H18N2O3�����ڵ����� | |
| B�� | ˮ������������ְ����� | |
| C�� | ��һ�������¼������ᷴӦ������Ӧ | |
| D�� | �������б������������Ȼ����ļ������Ľṹ |
| A�� | Na2O��ˮ��Ӧ��O2-+H2O�T2OH- | |
| B�� | SO2ʹ��ˮ��ɫ��SO2+Cl2+2H2O�T4H++SO42-+2Cl- | |
| C�� | Na2SiO3��Һ�����ᷴӦ��Na2SiO3+2H+�TH2SiO3��+2Na+ | |
| D�� | Ca��HCO3��2��Һ�����NaOH��Һ��Ӧ��Ca2++HCO3-+OH-�TCaCO3��+2H2O |
| A�� | CaCl2 | B�� | KCl | C�� | H2O | D�� | NH4NO3 |
| A�� | C2H5OH+CH3COOH$��_{��}^{Ũ����}$CH3COOC2H5+H2O | |
| B�� | H-C��C-H+HCl$\stackrel{����}{��}$H2C=CHCl | |
| C�� | 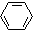 +HNO3$��_{60��}^{Ũ����}$ +HNO3$��_{60��}^{Ũ����}$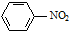 +H2O +H2O | |
| D�� |  +Br2$\stackrel{FeBr_{3}}{��}$ +Br2$\stackrel{FeBr_{3}}{��}$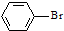 +HBr +HBr |
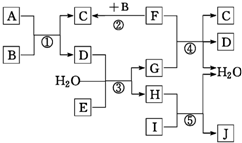 ����ת����ϵͼ�У�A��B��C��D��E�ڳ�����Ϊ���壬B��E��FΪ���ʣ�����FΪ��ɫ���壻C��D����ʹ�����ʯ��ˮ����ǣ�D��E����ʹƷ����Һ��ɫ��I��һ�ֺ���ɫ�����������Ӧ���й�̬F��G��Ũ��Һ�ڼ���ʱ���ܷ�����Ӧ���ش��������⣺
����ת����ϵͼ�У�A��B��C��D��E�ڳ�����Ϊ���壬B��E��FΪ���ʣ�����FΪ��ɫ���壻C��D����ʹ�����ʯ��ˮ����ǣ�D��E����ʹƷ����Һ��ɫ��I��һ�ֺ���ɫ�����������Ӧ���й�̬F��G��Ũ��Һ�ڼ���ʱ���ܷ�����Ӧ���ش��������⣺ ��
�� D����ϩ��1-��ϩ E��������2��3-��������
D����ϩ��1-��ϩ E��������2��3-��������