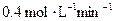��Ŀ����
(6��)��һ�ܱյ�2 L������װ��4 mol SO2��2 mol O2����һ�������¿�ʼ��Ӧ��2 minĩ�ﵽƽ���Ҳ����������1.6 mol SO2 ��
��1��2 minĩSO2��Ũ��_______________________________��
��2��2 min��SO2��ƽ����Ӧ����________________________��
(3)�ﵽƽ��ı�־��
������2 mol SO2��ͬʱ����2 mol SO3
�� SO2��O2��SO3�����ʵ���֮��Ϊ2��1��2
�۷�Ӧ������У�SO3�������������ٸı�
��1��2 minĩSO2��Ũ��_______________________________��
��2��2 min��SO2��ƽ����Ӧ����________________________��
(3)�ﵽƽ��ı�־��
������2 mol SO2��ͬʱ����2 mol SO3
�� SO2��O2��SO3�����ʵ���֮��Ϊ2��1��2
�۷�Ӧ������У�SO3�������������ٸı�
1.2mol/L 0.6mol/(L��min) ��
��

��ϰ��ϵ�д�
�����Ŀ
 2SO3(g)�����е������仯��ͼ��ʾ���ش��������⡣
2SO3(g)�����е������仯��ͼ��ʾ���ش��������⡣ SO2��1molO2ͨ�����Ϊ2L�ĺ��º����ܱ������У�������Ӧ��2minʱ��Ӧ�ﵽƽ�⣬��ʱ��÷�Ӧ��O2��ʣ��0.1mol , ��ﵽƽ��ʱSO2��ת����Ϊ ��(1��)
SO2��1molO2ͨ�����Ϊ2L�ĺ��º����ܱ������У�������Ӧ��2minʱ��Ӧ�ﵽƽ�⣬��ʱ��÷�Ӧ��O2��ʣ��0.1mol , ��ﵽƽ��ʱSO2��ת����Ϊ ��(1��)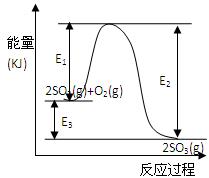
 SO3
SO3 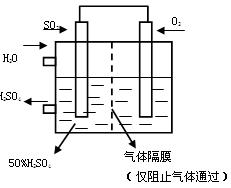
 O2��ˮ��������Ϊ (2��)
O2��ˮ��������Ϊ (2��)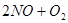

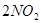 �ﵽƽ��ı�־��
�ﵽƽ��ı�־��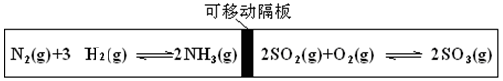
 �����ƶ���T��ʱ��M��
�����ƶ���T��ʱ��M�� N���������о�������ӦN2��g��+3H2��g��
N���������о�������ӦN2��g��+3H2��g�� 2NH3��g����������M��N�и�����l mol N2��3 mol H2����ʼM��N���ݻ����¶���ͬ���������¶Ȳ��䡣�����й�˵���в���ȷ���� �� ��
2NH3��g����������M��N�и�����l mol N2��3 mol H2����ʼM��N���ݻ����¶���ͬ���������¶Ȳ��䡣�����й�˵���в���ȷ���� �� ��

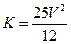
 3B(?)+C(?)����ʼ�������г���A,���ŷ�Ӧ�Ľ��У�����ƽ����Է���������С���������ж���ȷ����
3B(?)+C(?)����ʼ�������г���A,���ŷ�Ӧ�Ľ��У�����ƽ����Է���������С���������ж���ȷ����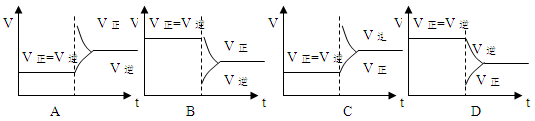
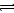 2Z(g)����֪X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.1 mol/L��0.3 mol/L��0.2 mol/L����һ�������£�����Ӧ�ﵽƽ��ʱ�����ʵ�Ũ�ȿ�����(����)
2Z(g)����֪X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.1 mol/L��0.3 mol/L��0.2 mol/L����һ�������£�����Ӧ�ﵽƽ��ʱ�����ʵ�Ũ�ȿ�����(����)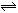 2C��������2min��Ӧ�ﵽƽ��ʱ����û�����干3.4 mol������0.4 mol C�������м�������ȷ����
2C��������2min��Ӧ�ﵽƽ��ʱ����û�����干3.4 mol������0.4 mol C�������м�������ȷ����