��Ŀ����
����β����NOx�����������������������㷺��ע��
��1��ij��ȤС����������������Ϣ��
N2(g)+O2(g)=2NO(g) ��H=+180.5kJ/mol
2H2(g)+O2(g)=2H2O(g)) ��H=�D483.6kJ/mol
��Ӧ2H2(g)+2NO(g)=2H2O(g)+N2(g) ��H= ��
��2����С�����õ��ԭ���������ͼ1װ�ý���H2��ԭNO��ʵ��[�����ӵ����Ե�SCY�մ�(�ܴ���H+)Ϊ���ʣ������ٱ�Ĥ���缫]��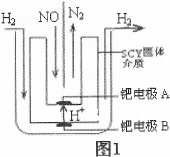
�ٵ缫AΪ �����缫��ӦʽΪ ��
��3����������ԭNOԭ�����£�
����Ӧ��4NO(g)+4NH3(g)+O2(g) 4N2(g)+6H2O(g) (��H <0)
4N2(g)+6H2O(g) (��H <0)
����Ӧ��4NH3(g)+3O2(g) 2N2(g)+6H2O(g)
2N2(g)+6H2O(g)
4NH3(g)+ 4O2(g) 2N2O(g)+6H2O(g)
2N2O(g)+6H2O(g)
4NO(g)+4NH3(g)+3O2(g) 4N2O(g)+6H2O(g)
4N2O(g)+6H2O(g)
�й�ʵ�������ͼ2��ͼ3��ʾ���ݴ˻ش��������⣺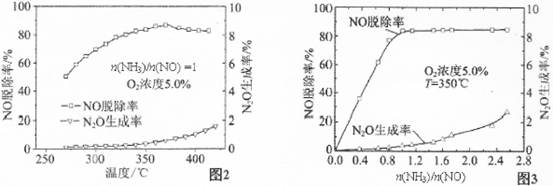
�ٴ���ԭNOӦ����n(NH3)/n(NO)�����ֵΪ �������� ��
������Ӧƽ�ⳣ������ʽ��K= �������¶ȵ����ӣ�K�� (ѡ����ӡ��� ����С�����䡱��
��Ӱ��N2O�����ʵ������� ������Ũ�Ⱥ� ��
��1���D664.1kJ/mol (2��)
��2������(2��) 2NO+4H++4e��=N2��+2H2O (2��)
��3����1 (1��) n(NH3)/n(NO)С��1ʱ��NO�ѳ��ʲ��ߣ�n(NH3)/n(NO)����1ʱ��NO�ѳ������Ӳ�������N2O�������������ӣ� 3�֣�
�� (2��) ��С(2��)
(2��) ��С(2��)
���¶�(1��) n(NH3)/n(NO) (1��)(�������մ�˳��ɵߵ�)
���������������1�����ݸ�˹���ɣ�����2����Ӧ��ȥ��1����Ӧ�ɵã���H=�D664.1kJ/mol����2����ԭ���ԭ����֪���ڵ�·��������������������ͼ��֪���ٵ缫AΪ�������ٵ缫BΪ�������ɵ��ӡ���ɡ�ԭ�Ӿ��غ��֪�����Ի��������������Ļ�ԭ��ӦʽΪ2NO+4H++4e��=N2��+2H2O����3���ٶ�ͼ���Աȿ�֪��n(NH3)/n(NO)С��1ʱ��NO�ѳ��ʲ��ߣ�n(NH3)/n(NO)����1ʱ��NO�ѳ������Ӳ�������N2O�������������ӣ����n(NH3)/n(NO)�����ֵΪ1�����ɷ�Ӧʽ��֪��K= �����ڡ�H<0������Ӧ���ȣ�����ƽ�����ƣ�K��С�����������Ϣ��֪��Ӱ��N2O�����ʵ��������¶ȡ�n(NH3)/n(NO) ������Ũ�ȡ�
�����ڡ�H<0������Ӧ���ȣ�����ƽ�����ƣ�K��С�����������Ϣ��֪��Ӱ��N2O�����ʵ��������¶ȡ�n(NH3)/n(NO) ������Ũ�ȡ�
���㣺�����˹���ɡ�ԭ��ء���ѧƽ������֪ʶ��

ij������ȼ���ɼס��������л����϶��ɣ��ס����������ʺ���C��H��O����Ԫ���е����ֻ����֡���֪�ס��Ҽ�CO��H2��ȼ�������£�
| ���� | �� | �� | CO | H2 |
| ȼ����/(kJ��mol��1) | 1 366 | 5 518 | 283 | 286 |
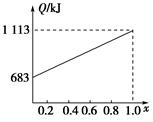
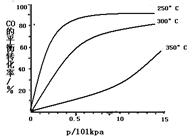
ȡ�ס��Ұ���ͬ������ϵ�ȼ��23 g����������O2��ȼ��ʱ���ų�������Q���������ҵ����ʵ�������x�Ĺ�ϵ��ͼ��ʾ������
(1)�ҵ���Է�������Mr(��)��________��
(2)160 g�ɼס����Ե����ʵ�����϶��ɵ�ȼ����347.2 L O2��ǡ����ȫȼ�գ���492.8 L���壬��ȴ������ʱ����ʣ��224 L(����������ڱ�״���²ⶨ)���ɴ˿���û�����У�C��H��O��ԭ�Ӹ�����Ϊ________���ס��ҵķ���ʽΪ����________����________��
(3)1 mol�ɼס����Ե����ʵ�����϶��ɵ�ȼ����һ������O2��ȼ�գ��ų�����2 876 kJ����Ӧ������CO________mol��
��1����֪��Ӧ�����������л�ѧ������ʱ�������仯������ʾ��
H2(g)��Cl2(g)��2HCl(g) ��H����184kJ/mol
4HCl (g)��O2(g)��2Cl2 (g) ��2H2O (g) ��H����115.6kJ/mol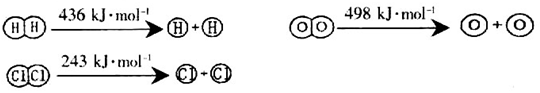
��H2��O2��Ӧ������̬ˮ���Ȼ�ѧ����ʽΪ_________________________________��
�ڶϿ�1mol H��O����������ԼΪ_________________________kJ��
��2����֪ij��Ӧ��ƽ�ⳣ������ʽΪ��K�� ,������Ӧ�Ļ�ѧ����ʽΪ________________��
,������Ӧ�Ļ�ѧ����ʽΪ________________��
��3����֪��ӦN2(g)��3H2(g) 2NH3(g) ��H��0��400��ʱK��0.5������������0.5L���ܱ������н��и÷�Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��Ӧ��(N2)��______��(N2)�棨�����������������������ʹ�ø÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬ͬʱʹƽ��ʱNH3������ٷ������ӣ��ɲ�ȡ�Ĵ�ʩ��_______������ţ���
2NH3(g) ��H��0��400��ʱK��0.5������������0.5L���ܱ������н��и÷�Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��Ӧ��(N2)��______��(N2)�棨�����������������������ʹ�ø÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬ͬʱʹƽ��ʱNH3������ٷ������ӣ��ɲ�ȡ�Ĵ�ʩ��_______������ţ���
A.��С�������ѹǿ B.�����¶�
C.�Ӵ��� D.ʹ����Һ������
��4����һ��������ܱ������н������»�ѧ��Ӧ��A(g)��3B(g) 2C(g)��D(s) ��H���仯ѧƽ�ⳣ��K��T�Ĺ�ϵ���±���
2C(g)��D(s) ��H���仯ѧƽ�ⳣ��K��T�Ĺ�ϵ���±���
| T/K | 300 | 400 | 500 | ���� |
| K/(mol��L��1)2 | 4��106 | 8��107 | 1.2��109 | ���� |
����һ�������£����жϸ÷�Ӧһ���ﻯѧƽ��״̬����______������ţ���
A.3 ��(B)����2��(C)�� B.A��B��ת�������
C.������ѹǿ���ֲ��� D.���������ܶȱ��ֲ���
�����γɶ����������NO��NO2��N2O4�ȡ���֪NO2��N2O4�Ľṹʽ�ֱ���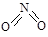 ��
��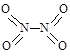 ��ʵ����N��N������Ϊ167kJ��mol��1�� NO2�е�������ƽ������Ϊ466 kJ��mol��1��N2O4�е�������ƽ������Ϊ438.5 kJ��mol��1��
��ʵ����N��N������Ϊ167kJ��mol��1�� NO2�е�������ƽ������Ϊ466 kJ��mol��1��N2O4�е�������ƽ������Ϊ438.5 kJ��mol��1��
��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ��
��2���Է�ӦN2O4(g) 2NO2(g)�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����
2NO2(g)�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���� 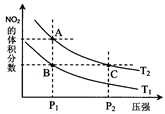
A��A��C����ķ�Ӧ���ʣ�A��C
B��B��C����������ƽ����Է���������B��C
C��A��C�����������ɫ��A�Cdz
D����״̬B��״̬A�������ü��ȵķ���
��3����100��ʱ����0.40mol��NO2�������2 L��յ��ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ����±����ݣ�
| ʱ�䣨s�� | 0 | 20 | 40 | 60 | 80 |
| n(NO2)/mol | 0.40 | n1 | 0.26 | n3 | n4 |
| n(N2O4)/mol | 0.00 | 0.050 | n2 | 0.080 | 0.080 |
�������������£��ӷ�Ӧ��ʼֱ��20 sʱ������������ƽ����Ӧ����Ϊ
��n3 n4���>������<����=�������÷�Ӧ��ƽ�ⳣ��K��ֵΪ �������¶Ⱥ�Ӧ2NO2
 N2O4��ƽ�ⳣ��K�� �����������С�����䡱����
N2O4��ƽ�ⳣ��K�� �����������С�����䡱������������ͬ����������������������N2O4���壬Ҫ�ﵽ����ͬ����ƽ��״̬��N2O4����ʼŨ����_____________mol��L��1��
��15�֣����Ŵ�����Ⱦ���������أ��������ڡ�ʮ���塱�ڼ䣬����������(SO2)�ŷ�������8%����������(NOx)�ŷ�������10%��Ŀǰ������������Ⱦ�ж��ַ�����
(1)��CH4����ԭ��������������������������Ⱦ����֪��
��CH4(g)+4NO2(g)��4NO(g) + CO2(g) +2H2O(g) �SH�� -574 kJ��mol��1
��CH4(g) +4NO(g)��2N2(g) + CO2(g) + 2H2O(g) �SH��-1160 kJ��mol��1
��H2O(g)��H2O(l) ��H��-44.0 kJ��mol��1
д��CH4(g)��NO2(g)��Ӧ����N2 (g)��CO2 (g)��H2O(1)���Ȼ�ѧ����ʽ ��
��2������Fe2+��Fe3+�Ĵ����ã������¿ɽ�SO2ת��ΪSO42-���Ӷ�ʵ�ֶ�SO2����������֪��SO2�ķ���ͨ�뺬Fe2+��Fe3+����Һʱ������һ����Ӧ�����ӷ���ʽΪ4Fe2+ + O2+ 4H+ ��4Fe3+ + 2H2O������һ��Ӧ�����ӷ���ʽΪ ��
(3)�û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2(g)+CO2(g)��ij�о�С�����ܱյ���������У���������������䣬��������������Բ��ƣ�����NO�������Ļ���̿������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)+CO2(g)��ij�о�С�����ܱյ���������У���������������䣬��������������Բ��ƣ�����NO�������Ļ���̿������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
Ũ��/mol��L��1
| NO | N2 | CO2 | ||
| 0 | 1.00 | 0 | 0 | ||
| 10 | 0.58 | 0.21 | 0.21 | ||
| 20 | 0.40 | 0.30 | 0.30 | ||
| 30 | 0.40 | 0.30 | 0.30 | ||
| 40 | 0.32 | 0.34 | 0.17 | ||
| 50 | 0.32 | 0.34 | 0.17 |
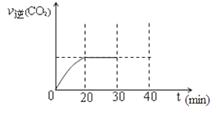
��10min��20min��v(CO2) ��ʾ�ķ�Ӧ����Ϊ ��
�ڸ��ݱ������ݣ�����T1��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ ���� ��������λС������
��һ���¶��£�����NO����ʼŨ��������NO��ƽ��ת���� ������������䡱��С���� ��
�����и�������Ϊ�жϸ÷�Ӧ�ﵽƽ����� ������ ���������ĸ����
A��������ѹǿ���ֲ��䡡�� B��2v��(NO)��v��(N2)
C��������CO2������������� D�����������ܶȱ��ֲ���
��30minĩ�ı�ijһ��������һ��ʱ�䷴Ӧ���´ﵽƽ�⣬��ı������������ ��������ͼ�л���30min��40min�ı仯���ߡ�
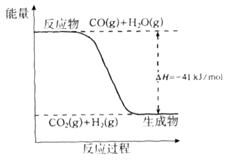
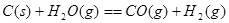
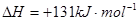

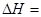
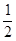 O2(g)��CO(g)��2H2(g) ��H2=��35.4 kJ��mol-1
O2(g)��CO(g)��2H2(g) ��H2=��35.4 kJ��mol-1 [Cu(NH3)3]Ac��CO ��H��0
[Cu(NH3)3]Ac��CO ��H��0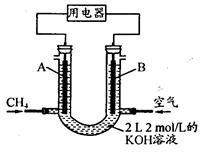
 7N2��12 H2OҲ�ɴ���NO2����ת��1.2mol����ʱ�����ĵ�NO2�ڱ�״������ L��
7N2��12 H2OҲ�ɴ���NO2����ת��1.2mol����ʱ�����ĵ�NO2�ڱ�״������ L�� 2SO3��g�� ��H="-196.6" kJ·mol-1
2SO3��g�� ��H="-196.6" kJ·mol-1