��Ŀ����
ij������ȼ���ɼס��������л����϶��ɣ��ס����������ʺ���C��H��O����Ԫ���е����ֻ����֡���֪�ס��Ҽ�CO��H2��ȼ�������£�
| ���� | �� | �� | CO | H2 |
| ȼ����/(kJ��mol��1) | 1 366 | 5 518 | 283 | 286 |
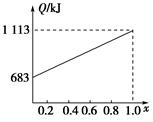
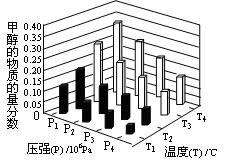
ȡ�ס��Ұ���ͬ������ϵ�ȼ��23 g����������O2��ȼ��ʱ���ų�������Q���������ҵ����ʵ�������x�Ĺ�ϵ��ͼ��ʾ������
(1)�ҵ���Է�������Mr(��)��________��
(2)160 g�ɼס����Ե����ʵ�����϶��ɵ�ȼ����347.2 L O2��ǡ����ȫȼ�գ���492.8 L���壬��ȴ������ʱ����ʣ��224 L(����������ڱ�״���²ⶨ)���ɴ˿���û�����У�C��H��O��ԭ�Ӹ�����Ϊ________���ס��ҵķ���ʽΪ����________����________��
(3)1 mol�ɼס����Ե����ʵ�����϶��ɵ�ȼ����һ������O2��ȼ�գ��ų�����2 876 kJ����Ӧ������CO________mol��
�� (1)114��(2)10��24��1��C2H6O��C8H18��(3)2
����

��ϰ��ϵ�д�
 ÿ�α���ϵ�д�
ÿ�α���ϵ�д�
�����Ŀ
��֪��Ӧ��Fe(s)+CO2(g) FeO(s)+CO(g)����H="a" kJ��mol-1,ƽ�ⳣ��ΪK;��Ӧ��CO(g)+1/2O2(g)
FeO(s)+CO(g)����H="a" kJ��mol-1,ƽ�ⳣ��ΪK;��Ӧ��CO(g)+1/2O2(g) CO2(g)����H="b" kJ��mol-1;��Ӧ��Fe2O3(s)+3CO(g)
CO2(g)����H="b" kJ��mol-1;��Ӧ��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H="c" kJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
2Fe(s)+3CO2(g)����H="c" kJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
| �¶�/�� | 500 | 700 | 900 |
| K | 1.00 | 1.47 | 2.40 |
(1)��500 ��ʱ���з�Ӧ��,CO2����ʼŨ��Ϊ2 mol��L-1,CO��ƽ��Ũ��Ϊ����������
(2)��Ӧ��Ϊ��������(ѡ����ȡ����ȡ�)��Ӧ��
(3)700 ��ʱ��Ӧ�ٴﵽƽ��״̬,Ҫʹ��ƽ�������ƶ�,������������ʱ,���Բ�ȡ�Ĵ�ʩ����������(�����)��
A.��С��Ӧ����� B.ͨ��CO2 C.�¶����ߵ�900 �� D.ʹ�ú��ʵĴ���
E.����Fe����
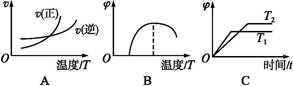
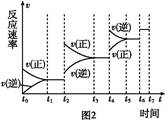
(4)����ͼ����Ϸ�Ӧ�ٵ�����������(�����)(ͼ��vΪ����,��Ϊ�������CO����,TΪ�¶���T1>T2)��
(5)�ɷ�Ӧ�ٺ͢ڿ����,��Ӧ2Fe(s)+O2(g)
 2FeO(s)�Ħ�H=����������
2FeO(s)�Ħ�H=���������� (6)�����ø�˹����д��Fe(����)��O2(����)�����õ�Fe2O3(����)���Ȼ�ѧ����ʽ:����
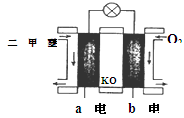
 CH3OCH3��H2O
CH3OCH3��H2O CH3OCH3(g)��CO2(g) ��H����247kJ/mol
CH3OCH3(g)��CO2(g) ��H����247kJ/mol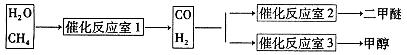
 2SO3(g)��
2SO3(g)��
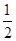 O2��g��=CO2��g���� ��H2��b kJ��mol��1
O2��g��=CO2��g���� ��H2��b kJ��mol��1 2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������
2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������ ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��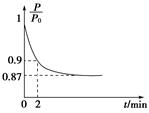
 CH3OH(g) ��H
CH3OH(g) ��H
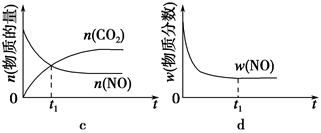
 2CO2��g����N2��g�������ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯������ͼ��ʾ��
2CO2��g����N2��g�������ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯������ͼ��ʾ��

 N2O4��g������H2����56.9 kJ��mol��1
N2O4��g������H2����56.9 kJ��mol��1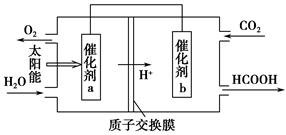
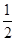 O2(g)===H2O(l) ��H3����285.84 kJ��mol��1
O2(g)===H2O(l) ��H3����285.84 kJ��mol��1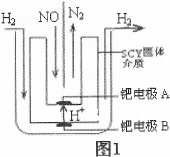
 4N2(g)+6H2O(g) (��H <0)
4N2(g)+6H2O(g) (��H <0)