��Ŀ����
����Ŀ�����������(LiFePO4)����������ӵ���������ϡ� LiFePO4����FeCl3��NH4H2PO4��LiCl�ͱ���(![]() )����Ϊԭ���Ʊ���
)����Ϊԭ���Ʊ���
(1) Fe2����̬��������Ų�ʽΪ________��PO43-�Ŀռ乹��Ϊ________(����������)��
(2) NH4H2PO4�У�����Ԫ���⣬��������Ԫ�ص�һ������������____(��Ԫ�ط���)��
(3) 1 mol![]() ���е�������ĿΪ___�������ķе���ڼױ�����Ҫԭ����________��
���е�������ĿΪ___�������ķе���ڼױ�����Ҫԭ����________��
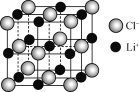
(4) һ��LiCl����(��ͼ)�У�Li����ĿΪ________��
���𰸡�1s22s22p63s23p63d6��[Ar]3d6 �������� N 14 mol �������γɷ��Ӽ���������ױ����Ӽ�û����� 4
��������
��1��Fe��26��Ԫ�أ���������Ų�ʽΪ1s22s22p63s23p63d64s2��[Ar]3d64s2��Fe2+��Feʧȥ���������γɵģ���Fe2+�ĺ�������Ų�ʽΪ1s22s22p63s23p63d6��[Ar]3d6��PO43-����ԭ�ӵŵ��Ӷ���Ϊ![]() ������ԭ�ӵ�����Ϊ4������PO43-����ԭ�ӵļ۲���Ӷ���Ϊ4����PO43-�Ŀռ乹��Ϊ�������壻
������ԭ�ӵ�����Ϊ4������PO43-����ԭ�ӵļ۲���Ӷ���Ϊ4����PO43-�Ŀռ乹��Ϊ�������壻
��2��ͬ����Ԫ�ص�һ�����ܴ��ϵ������μ�С����N��P������A��Ԫ������p�����������һ�����ܴ��ڵ���A�壬��N��O������N��O��P��һ������������N��
��3��1���ӱ����� ����������C��C֮���������6����C��H֮���������5����C��N֮���������1����N��H֮���������2��������14����������1 mol
����������C��C֮���������6����C��H֮���������5����C��N֮���������1����N��H֮���������2��������14����������1 mol![]() ���е�������ĿΪ14 mol�������ͼױ����Ƿ��Ӿ��壬�����������а���������֮������γ���������ױ�����֮��û����������Ա����ķе���ڼױ���
���е�������ĿΪ14 mol�������ͼױ����Ƿ��Ӿ��壬�����������а���������֮������γ���������ױ�����֮��û����������Ա����ķе���ڼױ���
��4����ͼ��֪��Li���ھ��������Ϻ;����ڣ�ÿ���ⱻ4���������ã���ÿ����������Li������Ϊ![]() ����
����

����Ŀ��Ԫ���ƶ��⡣
���ݲ��ֶ�����Ԫ�ص���Ϣ�ش����⡣
Ԫ�� | Ԫ����Ϣ |
A | ��������VIIA�� |
B | ����������������3�� |
C | ԭ��������11 |
D | D3����Ne�ĵ�������ͬ |
��1��A��B��Ԫ�ط��ŷֱ�Ϊ_______��________��C��D��Ԫ�����Ʒֱ�Ϊ________��_______��B��D��Ԫ�������ڱ������ڵ�λ�÷ֱ���________��____________��
��2��д��B��C�ĵ����ڼ���ʱ�ķ�Ӧ����ʽ��_____________��
��3����ԭ�ӽṹ�ĽǶȷ�����Cԭ����Dԭ�ӵ�__________��ͬ��д��A�ĵ�����ˮ��Ӧ�����ӷ���ʽ_______________��D�ĵ�����C���������ˮ������Һ��Ӧ�Ļ�ѧ����ʽ__________��



