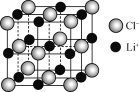
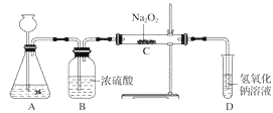
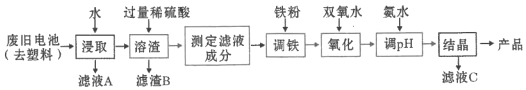
��Ŀ����
����Ŀ���Ȼ�����Ħ����������������Ҫ���࣬�ھ�ˮ�Ͷ��������ȷ��������Ҫ���á�
(1) ��FeCl3��Һ��ʴӡˢ��·���ϵ�ͭ����������Һ�м������ۣ��Լ������۳�ַ�Ӧ�����Һ������������____________��
a. ������ʣ�࣬����Һ��һ����Fe3+ b. ���й�����ڣ�����Һ��һ����Fe2+
c. ����Һ����Cu2+����һ��û�й������� d. ����Һ����Fe2+����һ����Cu����
(2)��ҵ��ͨ����������FeCl2��Һ�õ���FeCl3����Һ��������FeCl2��Һ�� ![]() ��
��![]() ��
��![]() �������Һ��
�������Һ��![]() ԼΪ______(������ˮ�ĵ�������ӵ�ˮ��)��
ԼΪ______(������ˮ�ĵ�������ӵ�ˮ��)��
(3)Ħ���� [(NH4)2Fe(SO4)26H2O]�ڶ��������г���Ϊ�����ʣ������궨�ظ���ء�������ص���Һ��Ũ�ȣ���![]() �ĸ��ε���Һ�У�����Ũ���ɴ�С��˳����____________��
�ĸ��ε���Һ�У�����Ũ���ɴ�С��˳����____________��
(4)Ħ���������Ը�����ط�����Ӧ��![]()
a.��ƽ�������ӷ���ʽ����___________��Fe2++��____________��MnO4-+��___________��H+����_________��Mn2++��__________��Fe3++��___________��H2O
b.��֪Ħ������Է�������Ϊ![]() ��ȡĦ���β�Ʒ
��ȡĦ���β�Ʒ![]() (���ʲ��������ط�Ӧ)�����
(���ʲ��������ط�Ӧ)�����![]() ��Һ��ȡ��
��Һ��ȡ��![]() ��
��![]() ��Һ�ζ�������
��Һ�ζ�������![]() ��Һ
��Һ![]() �����Ʒ��Ħ���ε���������Ϊ____________(��С����ʾ��������λС��)��
�����Ʒ��Ħ���ε���������Ϊ____________(��С����ʾ��������λС��)��
���𰸡�b 2 ![]() 5 1 8 1 5 4 0.8125
5 1 8 1 5 4 0.8125
��������
(1) FeCl3��Һ��ʴӡˢ��·���ϵ�ͭ������Ӧ��Cu+2Fe3+=Cu2++2Fe2+��Fe3+������ǿ��Cu2+��������������Fe3+��Ӧ������Cu2+��Ӧ���ݴ˷����жϣ�
(2)���ݵ���غ㣺c(Cl-)=2c(Fe2+)+3c(Fe3+)+c(H+)(������Һ��OH-Ũ�Ⱥ�С����������Ժ��Բ���)���ݴ���Һ�������ӵ�Ũ�ȣ��ٸ���pH=-lgc(H+)���㣻
(3)Ħ����[(NH4)2Fe(SO4)26H2O]�ܽ��γɵ���Һ�д���笠����ӡ��������ӡ���������ӣ�笠����ӡ���������ˮ�������ԣ��ݴ˷�������Ũ�ȴ�С��
(4)���ݻ��ϼ������غ�����жϷ���ʽ���ٸ������ĵĸ�����ؼ���25mL��Һ�к��е��������ӣ��Ӷ������Ʒ��Ħ���ε�����������
(1) a������ʣ�࣬˵��ͭȫ����ͭ������ʽ���ڣ����������ȫ��Ӧ����Һ��һ������Fe2+�����ܺ���Fe3+����a����b�����й�����ڣ�������һ����ͭ��������������Һ��һ������Fe2+����b��ȷ��c������Һ����Cu2+��������������㣬����ֻ��Fe3+��Ӧ��Ҳ������Fe3+��Ӧ��ʣ�ಿ����Cu2+��Ӧ����ͭ�����Կ����й�����������c����d���������������ʱ��ֻ����Fe+2Fe3+=3Fe2+ʱ��������ͭ��������d����ѡb��
(2)���ݵ���غ㣺c(Cl-)=2c(Fe2+)+3c(Fe3+)+c(H+)(������Һ��OH-Ũ�Ⱥ�С����������Ժ��Բ���)����c(H+)=c(Cl-)-2c(Fe2+)-3c(Fe3+)=5.3��10-2molL-1-2��2.0��10-2molL-1-3��1.0��10-3molL-1=1.0��10-2molL-1������ҺpH=-lg1.0��10-2=2���ʴ�Ϊ��2��
(3) Ħ����[(NH4)2Fe(SO4)26H2O]�ܽ��γɵ���Һ�д���笠����ӡ��������ӡ���������ӣ�笠����ӡ���������ˮ�⣬��Һ�����ԣ���ϻ�ѧʽ��Ũ����������������ӣ������笠����ӡ��������ӡ������Ӻ������������ӣ�����Ũ���ɴ�С��˳��Ϊ��c(SO42-)��c(NH4+)��c(Fe2+)��c(H+)��c(OH-)���ʴ�Ϊ��c(SO42-)��c(NH4+)��c(Fe2+)��c(H+)��c(OH-)��
(4)a.Fe2+��Fe3+����Ԫ�ػ��ϼ�����1�ۣ�MnO4-��Mn2+����Ԫ�ؽ���5�ۣ����ϼ�������С������Ϊ5����Fe2++ϵ��Ϊ5��MnO4- ϵ��Ϊ1������Ԫ���غ��֪Mn2+��Fe3+ϵ���ֱ�Ϊ1��H+ϵ��Ϊ2+3��5-[2��5-1]=8������HԪ���غ��֪H2Oϵ����4�����Է�Ӧ���ӷ���ʽΪ5Fe2++1MnO4-+8H+=1Mn2++5Fe3++4H2O���ʴ�Ϊ��5��1��8��1��5��4��
b.�����![]()
![]() ��Һ�����ĵĸ�����ص����ʵ���=0.0195L��0.05mol/L=0.000975mol������5Fe2++MnO4-+8H+=Mn2++5Fe3++4H2O֪����������Ϊ0.000975mol��5=0.004875mol����[(NH4)2Fe(SO4)26H2O]�����ʵ���Ϊ0.004875mol����˲�Ʒ��Ħ���ε���������Ϊ
��Һ�����ĵĸ�����ص����ʵ���=0.0195L��0.05mol/L=0.000975mol������5Fe2++MnO4-+8H+=Mn2++5Fe3++4H2O֪����������Ϊ0.000975mol��5=0.004875mol����[(NH4)2Fe(SO4)26H2O]�����ʵ���Ϊ0.004875mol����˲�Ʒ��Ħ���ε���������Ϊ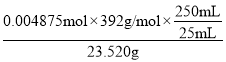 =0.8125���ʴ�Ϊ��0.8125��
=0.8125���ʴ�Ϊ��0.8125��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�