��Ŀ����
3��ij��ѧ�о�С���Cu�ij�������������ʽ���ʵ��̽�����о�������������£��������ڱ���Cu��Alλ�ñȽϽӽ���Al��OH��3�������ԣ�Cu��OH��2�Ƿ�Ҳ�����ԣ�
��ͨ������£�+2��Fe����ʮ3��Fe�ȶ���ʮl��CuҲһ������+2��Cu�ȶ���
��CuO�������ԣ��ܱ�H2��CO��ԭ��CuOҲ�ܱ�NH3��ԭ��
����Ϊ̽������٣���Cu��OH��2�����ѡ�õ��Լ�Ϊab������ţ���
a��ϡH2SO4 b��Ũ��NaOH��Һ c��Ũ��ˮ d��Ũ��Na2CO3��Һ
����Ϊ̽������ڣ���������ʵ�飺
��1����CuO��ĩ������1000��������ȫ�ֽ�ɺ�ɫ��Cu2O��ĩ���÷�Ӧ�Ļ�ѧ����ʽΪ4CuO$\frac{\underline{\;����\;}}{\;}$2Cu2O+O2�����÷�Ӧ˵�����ڸ��������£�+1�۵�Cu��+2��Cu���ȶ�����ȶ������ȶ�������
��2����Cu2O�м�����ϡ���ᣬ�õ���ɫ��Һ��һ�ֺ�ɫ���壬�ɴ˿�֪����������Һ�У�+1��Cu��+2��Cu�����ȶ�����ȶ������ȶ�������
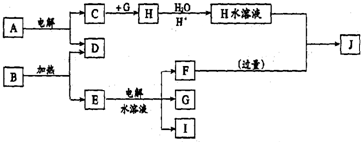
����Ϊ�������ۣ�ͬʱ�ⶨCu�Ľ������ԭ���������������������ʵ�飨�г�װ��δ������
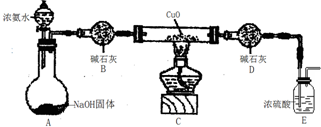
��1��װ��C��˵��CuO�ܹ���NH3��ԭ������Ϊ�����ɺ�ɫת���ɫ��NH3��CuO��Ӧ����ͭ��ͬʱ����һ������Ⱦ�����壬��Ӧ�Ļ�ѧ����ʽΪ3CuO+2NH3$\frac{\underline{\;����\;}}{\;}$N2+3Cu+3H2O��
��2���ⶨ��Ӧ��CuO��������H2O��������m��CuO����m��H2O�������г�����Cu�����ԭ�������ı���ʽ$\frac{18m��CuO��}{m��{H}_{2}O��}$-16��װ��D�����ԣ�����ԡ������ԡ�������װ��E��װ��E�����ã�������շ�Ӧ�����NH3����ֹ������ˮ�ֽ��룮
��3�����������ʹ�ⶨ���ƫ����ǣ�a������c����
��a��CuOδȫ����ԭΪCu ��b��CuO�ܳ� ��c��CuO�л���Cu��
���� ��I������֤��Al��OH��3�����Ե��Լ�ѡ��
��II����1������������ԭ��Ӧ�ж���һ����������ݷ�Ӧ��������Ӧ����д����Ӧ�ķ���ʽ������ת����ϵ�ж����ʵ��ȶ��ԣ�
��2������ת����ϵ�ж����ʵ��ȶ��ԣ�
��III����1������ͭΪ��ɫ���壬����ԭΪͭ���ʱ�Ϊ��ɫ������Ԫ���غ��������ж������ﲢд����Ӧ����ʽ��
��2�����������Ҫ������������ͭ��ˮ�����������ݰ����������ж�װ��E�����ã�
��3��Ҫʹ�ⶨ���ƫ����m��H2O��ҪƫС���ݴ˶Ը�ѡ������жϣ�
��� �⣺��I��֤��Al��OH��3�����Ե��Լ���ǿ���ǿ�Ҫ��֤��Cu��OH��2�����ԣ�Ҳ����ѡǿ���ǿ����Һ��������ǿ�ᣬ���Կ���ѡȡ������������ǿ����Կ�ѡȡ����ˮ��������Բ���ѡ����ѡa��b��
��II����1������������ԭ��Ӧ������ͭ���������ͻ�ԭ�����õ�������������ͭ��ʧ�����������������Է�Ӧ��������ͭ����������������ͭ����������Ӧ�����Ǹ��£����Է���ʽΪ��4CuO$\frac{\underline{\;����\;}}{\;}$2Cu2O+O2���� �����ɲ��ȶ�����ת��Ϊ�ȶ����ʵ����������ڸ��������£�ʮl�۵�Cu��ʮ2��Cu���ȶ���
�ʴ�Ϊ��4CuO$\frac{\underline{\;����\;}}{\;}$2Cu2O+O2�����ȶ���
��2�������ɲ��ȶ�����ת��Ϊ�ȶ����ʵ�����������������Һ�У�+1��Cu��+2��Cu�����ȶ����ʴ�Ϊ�����ȶ���
��III����1��װ��C�к�ɫ�����Ϊ��ɫ����֤��CuO��������ԭ������Ԫ���غ�������֪�������е������ǵ�����ͬʱ��ˮ���ɣ����Է�Ӧ����ʽΪ3CuO+2NH3$\frac{\underline{\;����\;}}{\;}$N2 ��+3Cu+3H2O���ʴ�Ϊ�������ɺ�ɫת���ɫ��3CuO+2NH3$\frac{\underline{\;����\;}}{\;}$N2+3Cu+3H2O����2����ͭ�����ԭ������Ϊx
2NH3+3CuO$\frac{\underline{\;����\;}}{\;}$N2+3Cu+3H2O
3��x+16��54
m��CuO�� m��H2O��
$\frac{3��x+16��}{m��CuO��}=\frac{54}{m��{H}_{2}O��}$�����x=$\frac{18m��CuO��}{m��{H}_{2}O��}$-16��Ũ����������ն���İ�������ֹ��Ⱦ�����������Է�ֹ������ˮ��������װ��D��Ӱ��ⶨ�����
�ʴ�Ϊ��$\frac{18m��CuO��}{m��{H}_{2}O��}$-16�������ԣ�������շ�Ӧ�����NH3����ֹ������ˮ�ֽ��룻
��3��Ҫʹ�ⶨ���ƫ����m��H2O��ҪƫС�����У�a������m��H2O��ƫС����b������m��H2O��ƫ��c���൱��m��H2O��ƫС����ѡ��a����c����
�ʴ�Ϊ����a����c����
���� ���⿼����Cu�ij�������������ʵ�ʵ��̽�������ü���ķ����ж�ͭ�ij�������������ʣ�Ȼ����ʵ����֤��Ҫע����ǣ����ж��������ʱ��һ����β������װ�ã�����ֱ���ſգ�

 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-������ͼ��ʾ��ϵ�����������������ӹ���˵������ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-������ͼ��ʾ��ϵ�����������������ӹ���˵������ȷ���ǣ�������| A�� | a���Ӧ����Һ�д������ڣ�Fe3+��Na+��SO42-��Cl- | |
| B�� | b���Ӧ����Һ�д������ڣ�Fe2+��Cl-��NO3-��Na+ | |
| C�� | c���Ӧ����Һ�д������ڣ�Ba2+��Cl-��Na+��Br- | |
| D�� | d���Ӧ����Һ�д������ڣ�Cu2+��K+��SO42-��NO3- |
| A�� | ����������������ˮ���� | |
| B�� | ��������������ˮ�ŷ���������Ȼˮ��������ķ��� | |
| C�� | �⻯���������˹����� | |
| D�� | ʹ�ú���ʳ�ο���Ԥ�������Ӳ��� |
��������Ƶ�ʯīϩ����������������̼���ࣩ��ѡ2013�����������������
��2014��11����Ѯ����APEC����ڼ䣬������ȭ������������������
���пƴ�����ɹ�������һ���µ������������� ��Li0.8Fe0.2��OHFeSe��Se��-2�ۣ�
�����ʼ�����ѧԺ��KTH�����о���Ա�Ѿ��ɹ��Ĺ�������һ��ʹˮ���ٷֽ�������ķ��Ӵ�����
����˵������ȷ���ǣ�������
| A�� | ʯīϩ������������������������ԭ��й©�����Ͳ��� | |
| B�� | �����к��д�����PM2.5��PM2.5�ֳ�Ϊ��ϸ����������������ж��к����ʣ��������ؽ��������������Σ�� | |
| C�� | �µ���������������Fe�Ļ��ϼ۳�+2��+3�� | |
| D�� | �÷��Ӵ����ɽ�һ����Դ̫����ֱ��ת���ɶ�����Դ |
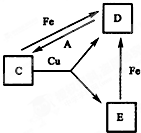 ��A��B��C��D��E��F���ֳ������ʣ������Ƿֱ�����ˮ��������Һ��ɫ������ͬ����֪��
��A��B��C��D��E��F���ֳ������ʣ������Ƿֱ�����ˮ��������Һ��ɫ������ͬ����֪��
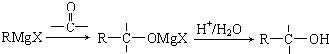
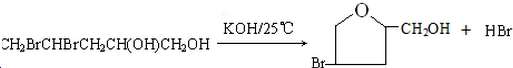 ����
����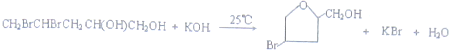 ������
������