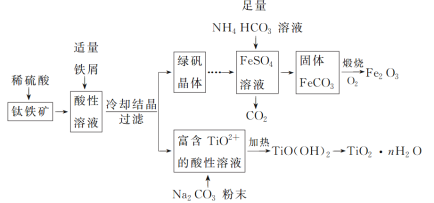
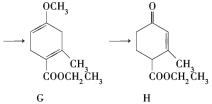
��Ŀ����
����Ŀ����֪�������£�(1)Ka1(H2CO3)=4.3��10-7��Ka2(H2CO3)=5.6��10-11��(2)H2R�������ε���Һ�У�H2R��HR-��R2-�ֱ�����������ռ�����ʵ�������(��)����ҺpH�ı仯��ϵ��ͼ��ʾ����������������ǣ� ��

A.��pH=1.3����Һ�У�c(Na+)��c(H2R)+2c(R2-)
B.�������Ũ�ȵ�NaOH��Һ��H2R��Һ��Ϻ���Һ��ˮ�ĵ���̶ȱȴ�ˮС
C.��pH=2����Һ�д���![]() =10-3
=10-3
D.��Na2CO3��Һ�м������H2R��Һ��������Ӧ��CO32-+H2R=HCO3-+HR-
���𰸡�D
��������
A����pH=1.3����Һ�У�c(H2R)=c(HR-)����Һ�е���غ�Ϊ��2c(R2-)+c(HR-)+c(OH-)=c(Na+)+c(H+)����Һ�����ԣ�c(OH-)��c(H+)����c(Na+)��2c(R2-)+c(HR-)=c(H2R)+2c(R2-)����A��ȷ��
B���������Ũ�ȵ�NaOH��Һ��H2R��Һ��Ϻ�����NaHR����ͼ��֪����Һ��HR-��R2-Ũ����ͬʱ��pH=4.3����Һ�����ԣ�˵��HR-�ĵ������ý�ǿ����ˮ�ĵ������������ã�������Һ��ˮ�ĵ���̶ȱȴ�ˮС����B��ȷ��
C������ҺpH=1.3ʱ��c(H2R)=c(HR-)����Ka1= 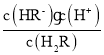 =10-1.3����Һ��pH=4.3ʱ��c(R2-)=c(HR-)����Ka2=
=10-1.3����Һ��pH=4.3ʱ��c(R2-)=c(HR-)����Ka2=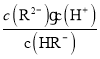 =10-4.3����pH=2����Һ�д���
=10-4.3����pH=2����Һ�д���![]() =
=![]() =10-3����C��ȷ��
=10-3����C��ȷ��
D����C�з���֪H2R�ĵ��볣��Ka2����H2CO3��Ka1�������ԣ�HR-��H2CO3��������Na2CO3��Һ�м������H2R��Һ��������Ӧ��CO32-+H2R=H2O+CO2��+R2-����D����
�ʴ�ΪD��

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�