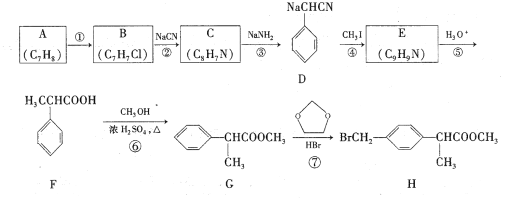
��Ŀ����
����Ŀ��������ᷢ����ʵ������Э�������ƶ���ɫ��̼ת�ͣ������������˹�ͬ��Ļ����źš���̬��ҵ��ѭ�����ó�Ϊ�ۺϽ��������Դ�������;��÷�չ��һ����Ч;����
��1��ˮ�ǡ�����֮���ʡ����ǡ���Զֵ��̽�������ʡ���
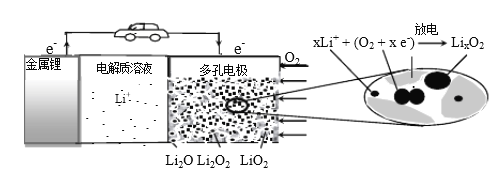
�Բ�������ʯī������Ƶ��أ�ͨ�����NH4HSO4��Һ����(NH4)2S2O8������ˮ��Ӧ�õ�H2O2���������ɵ�NH4HSO4����ѭ��ʹ�á�
�������ĵ缫��Ӧʽ��__��
���Ʊ�H2O2���ܷ�Ӧ����ʽ��__��
��2��CO2����Դ����������Ч����CO2�ŷţ��������̼��Դ��
CO2������ϳɶ�������һ��CO2ת�����������������Ҫ�������з�Ӧ��
��Ӧ��CO2(g)+H2(g)=CO(g)+H2O(g) ��H=41.2kJ��mol1
��Ӧ��2CO2(g)+6H2(g)=CH3OCH3(g)+3H2O(g) ��H=-122.5kJ��mol1
�ں�ѹ��CO2��H2����ʼ��һ���������£�CO2ƽ��ת���ʺ�ƽ��ʱCH3OCH3��ѡ�������¶ȵı仯��ͼ�����У�
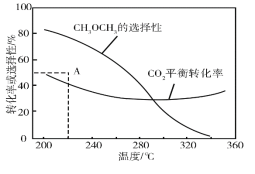
CH3OCH3��ѡ����=![]() ��100��
��100��
���¶ȸ���300�棬CO2ƽ��ת�������¶����߶�������ԭ����__��
��220��ʱ���ڴ���������CO2��H2��Ӧһ��ʱ����CH3OCH3��ѡ����Ϊ48%��ͼ��A�㣩�����ı䷴Ӧʱ����¶ȣ�һ�������CH3OCH3ѡ���ԵĴ�ʩ��__��
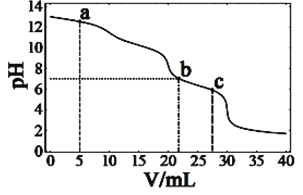
���������á���ͼװ�ü��Ա�Ҫ�ĵ������Ӻ�ﵽ���ô�ͭ����Ŀ�ġ�
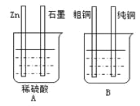
��A�ձ���__(����ء���ԭ��ء�)��
������Zn��B�ձ��е�__�������ͭ����ͭ������B�ձ���Ӧ��ʢ__��Һ��
�۷ֱ�д��ʯī���ʹ�ͭ���ĵ缫��Ӧʽ
ʯī����___��
��ͭ����__��
���𰸡�2HSO4-��2e-=S2O82��2H+(��2SO42-��2e-=S2O82) 2H2O![]() H2O2��H2�� ��Ӧ�����H��0����Ӧ�����H��0���¶�����ʹCO2ת��ΪCO��ƽ��ת����������ʹCO2ת��ΪCH3OCH3��ƽ��ת�����½������������ȳ����½����� ����ѹǿ��ʹ�öԷ�Ӧ������Ը��ߵĴ��� ԭ��� ��ͭ CuSO4�������������Ե�ͭ�Σ� 2H++2e-=H2�� Cu2++2e-=Cu
H2O2��H2�� ��Ӧ�����H��0����Ӧ�����H��0���¶�����ʹCO2ת��ΪCO��ƽ��ת����������ʹCO2ת��ΪCH3OCH3��ƽ��ת�����½������������ȳ����½����� ����ѹǿ��ʹ�öԷ�Ӧ������Ը��ߵĴ��� ԭ��� ��ͭ CuSO4�������������Ե�ͭ�Σ� 2H++2e-=H2�� Cu2++2e-=Cu
��������
(1)�ٵ��NH4HSO4��Һ�ù�����泥���ⷴӦ����ʽΪ��2NH4HSO4![]() (NH4)2S2O8��H2����������SO42����������S2O82����Һ�����ԣ��缫��ӦΪ2HSO4-��2e-=S2O82��2H+(��2SO42-��2e-=S2O82)���ʴ�Ϊ��2HSO4-��2e-=S2O82��2H+(��2SO42-��2e-=S2O82)��
(NH4)2S2O8��H2����������SO42����������S2O82����Һ�����ԣ��缫��ӦΪ2HSO4-��2e-=S2O82��2H+(��2SO42-��2e-=S2O82)���ʴ�Ϊ��2HSO4-��2e-=S2O82��2H+(��2SO42-��2e-=S2O82)��
��ͨ�����NH4HSO4��Һ����(NH4)2S2O8������ˮ��Ӧ�õ�H2O2���������ɵ�NH4HSO4����ѭ��ʹ�ã����NH4HSO4��Һ�ù�����泥���ⷴӦ����ʽΪ��2NH4HSO4![]() (NH4)2S2O8��H2����(NH4)2S2O8��Һ����ˮ�����м�ѹˮ�⡢�������������������ˮ��Һ��ʣ����Һ������������ѭ��ʹ�ã�˵��(NH4)2S2O8ˮ������˫��ˮ��������泥���Ӧ����ʽΪ(NH4)2S2O8��2H2O��2NH4HSO4��H2O2�����Ʊ�H2O2���ܷ�Ӧ����ʽ��2H2O
(NH4)2S2O8��H2����(NH4)2S2O8��Һ����ˮ�����м�ѹˮ�⡢�������������������ˮ��Һ��ʣ����Һ������������ѭ��ʹ�ã�˵��(NH4)2S2O8ˮ������˫��ˮ��������泥���Ӧ����ʽΪ(NH4)2S2O8��2H2O��2NH4HSO4��H2O2�����Ʊ�H2O2���ܷ�Ӧ����ʽ��2H2O![]() H2O2��H2�����ʴ�Ϊ��2H2O
H2O2��H2�����ʴ�Ϊ��2H2O![]() H2O2��H2����
H2O2��H2����
(2)���¶ȸ���300�棬CO2ƽ��ת�������¶����߶�������ԭ���Ƿ�Ӧ��ġ�H��0����Ӧ��ġ�H��0���¶�����ʹCO2ת��ΪCO��ƽ��ת����������ʹCO2ת��ΪCH3OCH3��ƽ��ת�����½������������ȳ����½����Ȼ�Ӧ��Ϊ���ȣ��� Ӧ��Ϊ���ȣ��¶ȸ���300��ʱ���Է�Ӧ��ռ�������ʴ�Ϊ����Ӧ�����H��0����Ӧ�����H��0���¶�����ʹCO2ת��ΪCO��ƽ��ת����������ʹCO2ת��ΪCH3OCH3��ƽ��ת�����½������������ȳ����½����ȣ�
��220��ʱ���ڴ���������CO2��H2��Ӧһ��ʱ����CH3OCH3��ѡ����Ϊ48%�����ı䷴Ӧʱ����¶ȣ�һ�������CH3OCH3ѡ���ԵĴ�ʩ������ѹǿ��ʹ�öԷ�Ӧ������Ը��ߵĴ������ʴ�Ϊ������ѹǿ��ʹ�öԷ�Ӧ������Ը��ߵĴ�����
(3)��A�ձ��������������Բ�ͬ�ĵ缫�����Է���������ԭ��Ӧ���γ��˱պϻ�·�����ڵ������Һ������ԭ���װ�ã�B�ձ��ʹ�������ӵ�Դ�����ڵ��װ�ã��ﵽ��ͭ������Ŀ�ģ��ʴ�Ϊ��ԭ��أ�
�ڵ�⾫��ͭʱ����ͭ�����������Դ��������������ͭ�����������Դ�ĸ����������������Һ�����Ǻ���ͭ���ӵĿ����Ե��Σ�������ͭ��Һ�����������Ե�ͭ�εȣ��ʴ�Ϊ����ͭ��CuSO4(�����������Ե�ͭ��)��
��ʯī����ԭ��ص������������缫��ӦΪ��2H����2e��H2������ͭ���ǵ��ص��������缫��ӦΪ��Cu2span>����2e��Cu���ʴ�Ϊ��2H����2e��H2����Cu2����2e��Cu��

����Ŀ������ʵ��������ʵ���������ƥ�����
ʵ����� | ʵ������ | |
A | ��ʢ�и������������Һ���Թ���ͨ����������ϩ���� | ��Һ����ɫ����ȥ�����ú���Һ�ֲ� |
B | ��þ����ȼ��Ѹ�����뼯��CO2�ļ���ƿ | ����ƿ�в���Ũ�̲��к�ɫ�������� |
C | ��ʢ�б��������������Һ���Թ��еμ�ϡ���� | �д̼�����ζ�����������Һ����� |
D | ��ʢ��FeCl3��Һ���Թ��мӹ������ۣ�������1��KSCN��Һ | ��ɫ����ʧ����KSCN����Һ��ɫ���� |
A. AB. BC. CD. D