��Ŀ����
12���±���Ԫ�����ڱ���һ���֣����a��kʮһ��Ԫ�ػش��������⣨���þ��廯ѧʽ�ش𣬷����֣���Ԫ�����ڱ�
| ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
| 2 | a | c | d | e | f | g | |
| 3 | b | h | i | j | k |
��2������K���⻯��Ļ�ѧʽΪHCl�����Ļ�ԭ�Ա�j���⻯�ﻹԭ�������ǿ��������������ˮ��Һ�����Ա�j���⻯��ˮ��Һ������ǿ���ǿ������������
��3��e����ΪN2����ṹʽΪN��N��
��4��д��i����������ǿ�Ӧ�����ӷ���ʽAl2O3+2OH-=2AlO2-+H2O��
���� ����Ԫ�������ڱ��е�λ��֪��a��k�ֱ���Li��Na��Be��B��N��O��F��Mg��Al��S��Cl��
��1��ͬһ����Ԫ�أ�Ԫ�طǽ���������ԭ�������������ǿ��ͬһ����Ԫ�أ��ǽ���������ԭ�����������������Ԫ�طǽ�����Խǿ��������������ˮ��������Խǿ����O��FԪ�س��⣻Ԫ�ؽ�����Խǿ��������������ˮ�������Խǿ��
��2��kΪClԪ�أ����⻯����HCl��Ԫ�صķǽ�����Խǿ�����⻯��Ļ�ԭ��Խ����
������ǿ�ᡢ����ˮ��Һ�����
��3��e�ĵ����ǵ���������������Nԭ��֮��������Թ��õ��Ӷԣ�
��4��i�������������������������������������ǿ������ƫ�����κ�ˮ��
��� �⣺����Ԫ�������ڱ��е�λ��֪��a��k�ֱ���Li��Na��Be��B��N��O��F��Mg��Al��S��Cl��
��1��ͬһ����Ԫ�أ�Ԫ�طǽ���������ԭ�������������ǿ��ͬһ����Ԫ�أ��ǽ���������ԭ��������������������Էǽ�������ǿ��Ԫ��λ�����ڱ����Ͻǣ�ϡ��������⣩���䵥��ΪF2��
Ԫ�طǽ�����Խǿ��������������ˮ��������Խǿ����O��FԪ�س��⣬ʣ����ЩԪ���зǽ�������ǿ����ClԪ�أ���������������ˮ����������ǿ����HClO4��
Ԫ�ؽ�����Խǿ��������������ˮ�������Խǿ���⼸��Ԫ�ؽ�������ǿ����Na�����Լ�����ǿ����NaOH��
�ʴ�Ϊ��F2��HClO4��NaOH��
��2��kΪClԪ�أ����⻯����HCl��Ԫ�صķǽ�����Խǿ�����⻯��Ļ�ԭ��Խ�����ǽ�����Cl��S�������⻯��Ļ�ԭ��HCl��H2S��
������ǿ�ᡢ����ˮ��Һ�����ᣬ����ˮ��Һ�����Ա�j���⻯��ˮ��Һ������ǿ��
�ʴ�Ϊ��HCl������ǿ��
��3��e�ĵ�����N2������������Nԭ��֮��������Թ��õ��Ӷԣ��ṹʽΪN��N��
�ʴ�Ϊ��N2��N��N��
��4��i�������������������������������������ǿ������ƫ�����κ�ˮ�����ӷ�Ӧ����ʽΪAl2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
���� ���⿼��Ԫ�����ڱ���Ԫ���������ۺ�Ӧ�ã��漰���ʽṹ�����ӷ�Ӧ��Ԫ�������ɵĿ��飬��ϤԪ�����ڱ��ṹ�����ӷ���ʽ��д���ɽ����Ŀ�ѶȲ���

| A�� | ����������л�ԭ�� | |
| B�� | ��Ϊ�����������Ư���ԣ���ʹƷ����Һ������ɫ | |
| C�� | �����ж�����̼Ũ�ȸ�ʱ���γ����� | |
| D�� | Ũ���᳣�������������ΪŨ���������ˮ�� |
| ѡ�� | ����� | ���롢�ᴿ���� |
| A�� | ���뱽��ˮ | ��Һ�� |
| B�� | �����Ҵ��ͼ״� | ���� |
| C�� | ��ȥ��Ȳ��H2S���� | ��CuSO4��Һϴ�� |
| D�� | ��ȥˮ�е��������� | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | NO��NO2 | B�� | CO��CO2 | C�� | CO2��SO2 | D�� | CH4��NH3 |
| A�� | �ձ� | B�� | ������ | C�� | ������ƿ | D�� | �Թ� |
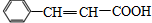
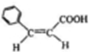 ��
��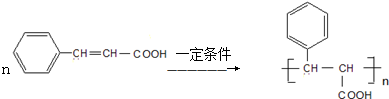 ��
�� ��
��