��Ŀ����
4��������A��C11H8O4��������������Һ�м��ȷ�Ӧ�����ữ�ɵõ�������B��C���ش��������⣺��1��B�ķ���ʽΪC2H4O2��������ֻ��һ�������ţ���B�Ľṹ��ʽ��CH3COOH��B���Ҵ���Ũ������¼��ȷ�Ӧ����D���÷�Ӧ�Ļ�ѧ����ʽ��CH3COOH+CH3CH2OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O��
��2��C�Ƿ��㻯�����Է�������Ϊ180����̼����������Ϊ60.0%�������������Ϊ4.4%������Ϊ������C�ķ���ʽ��C9H8O4��
��3����֪C�ķ�����������ȡ����������һ��ȡ������֧�����һ�����ʹ������Ȼ�̼��Һ��ɫ�Ĺ����ż�����̼��������Һ��Ӧ�ų�����Ĺ����ţ����ȡ�����ϵĹ�����������̼̼˫�����Ȼ�����������ȡ������ͬ���ֱ�λ�ڸ�ȡ��������λ�Ͷ�λ����C�Ľṹ��ʽ��
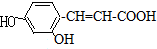 ��
��
���� ������A��C11H8O4��������������Һ�м��ȷ�Ӧ�����ữ�ɵõ�������B��C��˵��A�к���������B�ķ���ʽΪC2H4O2��������ֻ��һ�������ţ�����B�����ᣬ������Ҵ��ܷ���������Ӧ������������D��
C�Ƿ��㻯�����Է�������Ϊ180����̼����������Ϊ60.0%�������������Ϊ4.4%������Ϊ��������C��̼�����ĺ���ȷ�������ʽ��
C�ķ�����������ȡ����������һ��ȡ������֧�����һ�����ʹ������Ȼ�̼��Һ��ɫ�Ĺ����ż�����̼��������Һ��Ӧ�ų�����Ĺ����ţ�˵����֧���Ϻ���̼̼˫�����Ȼ�����������ȡ������ͬ���ֱ�λ�ڸ�ȡ��������λ�Ͷ�λ������C��ԭ�Ӹ���ȷ����ȡ�������ƣ���д����ṹ��ʽ��
��� ���⣺��1��������A��C11H8O4��������������Һ�м��ȷ�Ӧ�����ữ�ɵõ�������B��C��˵��A�к���������B�ķ���ʽΪC2H4O2��������ֻ��һ�������ţ�����B�Ľṹ��ʽ��CH3COOH���ڼ��ȡ�Ũ�������������Ҵ���Ӧ������������D���÷�Ӧ�Ļ�ѧ����ʽ��CH3COOH+CH3CH2OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3COOH��CH3COOH+CH3CH2OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O��
��2��C�Ƿ��㻯���˵��C�к��б�������Է�������Ϊ180����̼����������Ϊ60.0%����̼ԭ�Ӹ���Ϊ9�������������Ϊ4.4%������ԭ�Ӹ���Ϊ8������Ϊ������Oԭ�Ӹ���Ϊ4����C�ķ���ʽ��C9H8O4��
�ʴ�Ϊ��C9H8O4��
��3��C�ķ�����������ȡ����������һ��ȡ������֧�����һ�����ʹ������Ȼ�̼��Һ��ɫ�Ĺ�����˵������̼̼˫��������̼��������Һ��Ӧ�ų�����Ĺ����ţ�˵�������Ȼ�����������ȡ������ͬ�����ݷ���ʽ�������������Ĺ�����Ϊ�ǻ�����ֱ�λ�ڸ�ȡ��������λ�Ͷ�λ����C�Ľṹ��ʽ��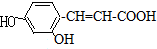 ��
��
�ʴ�Ϊ��̼̼˫�����Ȼ���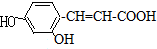 ��
��
���� ���⿼���л�����ƶϣ���ȷ���ʺ��еĹ����ż��������ǽⱾ��ؼ����ѵ���ͬ���칹�����д����Ŀ�Ѷ��еȣ�

| Ԫ�ش��� | X | Y | Z | W |
| ԭ�Ӱ뾶/pm | 160 | 143 | 75 | 74 |
| ��Ҫ���ϼ� | +2 | +3 | +3��+5��-3 | -2 |
| A�� | Y������������Ӧ��ˮ���������� | |
| B�� | һ�������£�Z������W�ij�������ֱ������ZW2 | |
| C�� | X��YԪ�صĽ����� X��Y | |
| D�� | X2+���Ӱ뾶����W2-���Ӱ뾶 |
Ԫ�����ڱ�
| ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
| 2 | a | c | d | e | f | g | |
| 3 | b | h | i | j | k |
��2������K���⻯��Ļ�ѧʽΪHCl�����Ļ�ԭ�Ա�j���⻯�ﻹԭ�������ǿ��������������ˮ��Һ�����Ա�j���⻯��ˮ��Һ������ǿ���ǿ������������
��3��e����ΪN2����ṹʽΪN��N��
��4��д��i����������ǿ�Ӧ�����ӷ���ʽAl2O3+2OH-=2AlO2-+H2O��
| A�� | ����ϩΪԭ���Ʊ��������� | B�� | �����������Ʊ�һ�ȼ��� | ||
| C�� | ��ͭ��Ũ����Ϊԭ����������ͭ | D�� | ��SiO2�Ʊ��ߴ��� |
| A | |||||||
| B | C | D | |||||
| E | F | G | |||||
��2���ֱ�д��BA4��EG�ĵ���ʽ
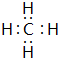 ��
��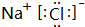
��3���õ���ʽ��ʾ���ӻ�����E2D��BD2���γɹ��̣�
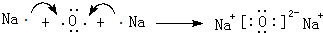 ��
��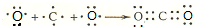
��4��F��G������������Ӧ��ˮ����ֱ���Al��OH��3��HClO4�����߷�Ӧ�����ӷ���ʽ��Al��OH��3+3H+=Al3++3H2O��
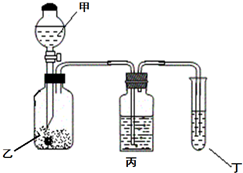
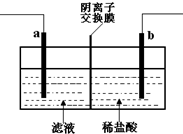 ��֪�����ӽ���Ĥֻ����������ͨ����ij��ѧ����С��������·�����ӡˢ��·��Һ��������Ҫ��FeCl2��CuCl2�� FeCl3�����������������Һ�м���������ۣ���ַ�Ӧ����ˣ��ٽ���Һת��ͼ��ʾ��װ���н��е�⣮����˵���в���ȷ���ǣ�������
��֪�����ӽ���Ĥֻ����������ͨ����ij��ѧ����С��������·�����ӡˢ��·��Һ��������Ҫ��FeCl2��CuCl2�� FeCl3�����������������Һ�м���������ۣ���ַ�Ӧ����ˣ��ٽ���Һת��ͼ��ʾ��װ���н��е�⣮����˵���в���ȷ���ǣ�������| A�� | ���ʱ���缫aӦ�����Դ���������� | |
| B�� | ���ʱ���缫b��Χ������ɫ��ζ������ | |
| C�� | ���ʱ���缫a�����ķ�Ӧ�ǣ�2Cl--2e-=Cl2�� | |
| D�� | ���ʱ���������Ȼ�����Һ���ϡ���� |
| A�� | CH3OH | B�� | 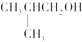 | C�� | 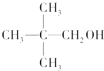 | D�� | 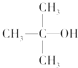 |
 ���ֶ�����Ԫ��A��B��C��D��E��ԭ��������������A��Cͬ�壬B��D ͬ�壬C���Ӻ�B���Ӿ�����ͬ�ĵ��Ӳ�ṹ��A��B��D��E�����γɹ����ͻ����A��B�γɵĻ�������ˮ�гʼ��ԣ�C��E�γɵĻ�������ˮ�г����ԣ��ش��������⣺
���ֶ�����Ԫ��A��B��C��D��E��ԭ��������������A��Cͬ�壬B��D ͬ�壬C���Ӻ�B���Ӿ�����ͬ�ĵ��Ӳ�ṹ��A��B��D��E�����γɹ����ͻ����A��B�γɵĻ�������ˮ�гʼ��ԣ�C��E�γɵĻ�������ˮ�г����ԣ��ش��������⣺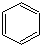
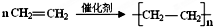 ��
��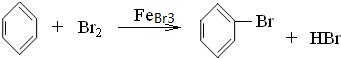 ��
��