��Ŀ����
��1�����й���ʵ�����������ȷ���� ������ĸ����
A����������ȼ�ŵľƾ���ʹ�ƾ���������ȼ��������Ӧ������ʪĨ����� |
B����������մ��Ƥ���������ϣ�Ӧ������ŨNaOH��Һ��ϴ |
C��������ƽ���������ϸ���һ����ͬ������ֽ���ٰ��������ƹ������ֽ�ϳ� |
D�����Լ�ƿ�е�Na2CO3��Һ�����Թ��У�����ȡ�����࣬Ϊ�˲��˷ѣ��ְѶ�����Լ�����ԭ�Լ�ƿ�� |
E����Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ���
F��ʹ�÷�Һ©��ǰҪ������Ƿ�©ˮ
G������������ʹNaCl����Һ������ʱ��Ӧ����������NaCl��Һ�е�ˮȫ����������
��2��ijѧУʵ���Ҵӻ�ѧ�Լ��̵����18.4 mol��L��1�����ᡣ
�ֽ���Ũ�������Ƴ�100 mL 1 mol��L��1��ϡ���ᡣ�ɹ�ѡ�õ������У�
a����ͷ�ι� b����ƿ c���ձ� d��ҩ�� e����Ͳ f��������ƽ
��ش��������⣺
�� ����ϡ����ʱ�����������в���Ҫʹ�õ��� ��ѡ����ţ�����ȱ�ٵ������� ��д�������ƣ���
�� ����100 mL 1 mol��L��1��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ mL������һλС��������ȡŨ����ʱӦѡ�� ������ţ�������Ͳ��
a��10 mL b��50 mL c��100 mL
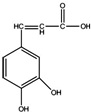
| A�� | ����ʽΪC9H5O4 | |
| B�� | 1mol������������5mol���������ӳɷ�Ӧ | |
| C�� | ����ˮ���ܷ���ȡ����Ӧ�����ܷ����ӳɷ�Ӧ | |
| D�� | ����Na2CO3��Һ��Ӧ����������NaHCO3��Һ��Ӧ |
| A�� | Ca��HCO3��2��NaOH��Һ��Ӧ | B�� | NaHCO3�����ʯ��ˮ��Ӧ | ||
| C�� | Ca��HCO3��2�����ʯ��ˮ��Ӧ | D�� | NH4HCO3�����ʯ��ˮ��Ӧ |
| A�� | �������۷ų�H2����Һ�У�Al3+��K+��SO42-��Cl- | |
| B�� | 1.0mol•L-1��KNO3��Һ�У�Na+��Fe2+��Cl-��SO42- | |
| C�� | �ں�����Fe3+����Һ�У�NH4+��Na+��Cl-��HCO3- | |
| D�� | ˮ���������c��OH-��=10-12 mol•L-1����Һ�У�K+��Cu2+��SO42-��NO3- |
| A�� | ��0.1mol•L-1Na2C2O4��Һ�У�2c��Na+��=c��C2O42-��+c��HC2O4-��+c��H2C2O4�� | |
| B�� | ��10mL pH=12��NaOH��Һ�еμӵ����pH=2��CH3COOH��Һ��c��CH3COO-����c��Na+����c��OH-����c��H+�� | |
| C�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1��С�մ���Һ���ռ���Һ�������ϣ�c��Na+��+c��H+��=2c��CO32-��+c��OH-��+c��HCO3-�� | |
| D�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1�����������Һ������������Һ��������c��SO42-��=c��Na+����c��NH4+����c��H+����c��OH-�� |


 $��_{��}^{-H_{2}O}$CH3CH=CHCHO
$��_{��}^{-H_{2}O}$CH3CH=CHCHO +CO$��_{��}^{AlCl_{3}��HCl}$
+CO$��_{��}^{AlCl_{3}��HCl}$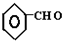

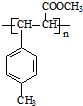 ��
�� ��
��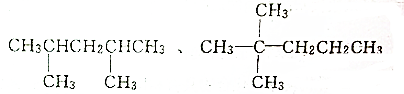
 ��NO
��NO
 ��NO
��NO