��Ŀ����
10�������£������й�������ȷ���ǣ�������| A�� | ��0.1mol•L-1Na2C2O4��Һ�У�2c��Na+��=c��C2O42-��+c��HC2O4-��+c��H2C2O4�� | |
| B�� | ��10mL pH=12��NaOH��Һ�еμӵ����pH=2��CH3COOH��Һ��c��CH3COO-����c��Na+����c��OH-����c��H+�� | |
| C�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1��С�մ���Һ���ռ���Һ�������ϣ�c��Na+��+c��H+��=2c��CO32-��+c��OH-��+c��HCO3-�� | |
| D�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1�����������Һ������������Һ��������c��SO42-��=c��Na+����c��NH4+����c��H+����c��OH-�� |
���� A���κε������Һ�ж����������غ㣬���������غ��жϣ�
B��pH=2�Ĵ���Ũ�ȴ���pH=12��NaOH������Ϻ������ʣ�࣬�������̶ȴ��ڴ��������ˮ��̶ȵ�����Һ�����ԣ�
C���κε������Һ�ж����ڵ���غ㣬���ݵ���غ��жϣ�
D��Ũ�Ⱦ�Ϊ0.1 mol•L-1�����������Һ������������Һ�������ϣ�����ǡ�÷�Ӧ���������ơ�����狀�ˮ�������ˮ�����Һ�����ԣ�
��� �⣺A���κε������Һ�ж����������غ㣬���������غ��c��Na+��=2c��C2O42-��+2c��HC2O4-��+2c��H2C2O4������A����
B��pH=2�Ĵ���Ũ�ȴ���pH=12��NaOH������Ϻ������ʣ�࣬�������̶ȴ��ڴ��������ˮ��̶ȵ�����Һ�����ԣ�����c��H+����c��OH-������B����
C������ǡ�÷�Ӧ����̼���ƣ��κε������Һ�ж����ڵ���غ㣬���ݵ���غ��c��Na+��+c��H+��=2c��CO32-��+c��OH-��+c��HCO3-������C��ȷ��
D��Ũ�Ⱦ�Ϊ0.1 mol•L-1�����������Һ������������Һ�������ϣ�����ǡ�÷�Ӧ���������ơ�����狀�ˮ�������ˮ�����Һ�����ԣ�笠�����ˮ��̶Ƚ�С����������غ��c��SO42-��=c��Na+����c��NH4+����c��H+����c��OH-������D��ȷ��
��ѡCD��
���� ���⿼������Ũ�ȴ�С�Ƚϣ�Ϊ��Ƶ���㣬��ȷ��Һ�е����ʼ������ʡ���Һ������ǽⱾ��ؼ���ע�����غ�������غ��������ã��״�ѡ����D��

��1�����й���ʵ�����������ȷ���� ������ĸ����
A����������ȼ�ŵľƾ���ʹ�ƾ���������ȼ��������Ӧ������ʪĨ����� |
B����������մ��Ƥ���������ϣ�Ӧ������ŨNaOH��Һ��ϴ |
C��������ƽ���������ϸ���һ����ͬ������ֽ���ٰ��������ƹ������ֽ�ϳ� |
D�����Լ�ƿ�е�Na2CO3��Һ�����Թ��У�����ȡ�����࣬Ϊ�˲��˷ѣ��ְѶ�����Լ�����ԭ�Լ�ƿ�� |
E����Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ���
F��ʹ�÷�Һ©��ǰҪ������Ƿ�©ˮ
G������������ʹNaCl����Һ������ʱ��Ӧ����������NaCl��Һ�е�ˮȫ����������
��2��ijѧУʵ���Ҵӻ�ѧ�Լ��̵����18.4 mol��L��1�����ᡣ
�ֽ���Ũ�������Ƴ�100 mL 1 mol��L��1��ϡ���ᡣ�ɹ�ѡ�õ������У�
a����ͷ�ι� b����ƿ c���ձ� d��ҩ�� e����Ͳ f��������ƽ
��ش��������⣺
�� ����ϡ����ʱ�����������в���Ҫʹ�õ��� ��ѡ����ţ�����ȱ�ٵ������� ��д�������ƣ���
�� ����100 mL 1 mol��L��1��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ mL������һλС��������ȡŨ����ʱӦѡ�� ������ţ�������Ͳ��
a��10 mL b��50 mL c��100 mL
| A�� | ���������������������ֱ���ȫȼ�գ����߷ų��������� | |
| B�� | ��C�����ʯ����C��ʯī��+119KJ ��֪�����ʯ��ʯī�ȶ� | |
| C�� | ��101Kpaʱ��2gH2��ȫȼ������Һ̬ˮ���ų�285.8KJ����������ȼ�յ��Ȼ�ѧ����ʽΪ��2H2��g��+O2��g����2H2O��l��+285.8KJ | |
| D�� | ��ϡ��Һ�У�H+��aq��+OH-��aq����H2O��l��+53.7KJ��������0.5 molH2SO4��Ũ��Һ�뺬1 molNaOH����Һ��ϣ��ų�����������53.7KJ |
| A�� | NaHSO3��Һ�У�c��SO32-����c��H2SO3�� | |
| B�� | ���չ����У�ʼ�մ����ţ�c��Na+��+c��H+��=c��OH-��+2c��SO32-��+c��HSO3-�� | |
| C�� | ������Һ�У�c��Na+����c��HSO3-��=c��SO32-����c��OH-��=c��H+�� | |
| D�� | ������Һ�У�c��Na+��=c��SO32-��+c��HSO3-�� |
 ������Ԫ��X��Y��Z��W�����ڱ��е����λ����ͼ��X����̬�⻯������������������Ӧ��ˮ���ﷴӦ�����Σ������жϴ�����ǣ�������
������Ԫ��X��Y��Z��W�����ڱ��е����λ����ͼ��X����̬�⻯������������������Ӧ��ˮ���ﷴӦ�����Σ������жϴ�����ǣ�������| A�� | �����̬�⻯����ȶ��ԣ�X��Y | |
| B�� | ���������Ӱ뾶��Z��W | |
| C�� | ��ZԪ�ص�����Һ���������ԡ����Ի����� | |
| D�� | WԪ�صĵ��ʾ���Ư���ԣ�����������ˮ��ɱ������ |
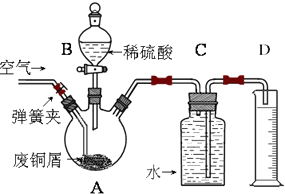 һѧϰС��������ͼ��ʾװ�ã���ij������Fe�ķ�ͭм����ͭ�����IJⶨ����̽���������Ʊ�����ͭ��Һ��
һѧϰС��������ͼ��ʾװ�ã���ij������Fe�ķ�ͭм����ͭ�����IJⶨ����̽���������Ʊ�����ͭ��Һ��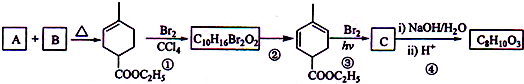
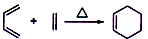
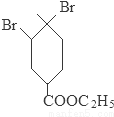 +2NaOH
+2NaOH
 +2NaBr+2H2O��
+2NaBr+2H2O��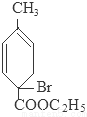 +2H2O
+2H2O
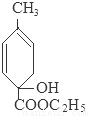 +C2H5OH+HBr��
+C2H5OH+HBr�� ��
��