��Ŀ����
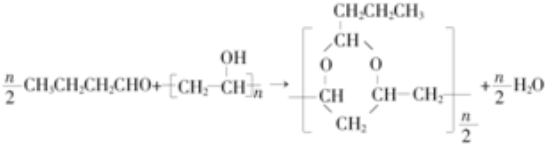
1���ϳɾ����������オ���Ե��л��߷��Ӳ������л���ѧ�о�����Ҫ����֮һ���۴�����ϩ���� PVAc��ˮ�����ɵľ���ϩ����PVA���������������オ���ԣ�������������ȫ�����в����PVB���йغϳ�·������ͼ�����ַ�Ӧ�����Ͳ�����ȥ����
��֪��
��
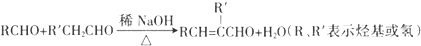
��
��ش�
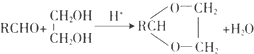
��1��AΪ����һԪ������������������ԼΪ34.8%��A�Ļ�ѧ����Ϊ�Ҵ���PVA�Ľṹ��ʽΪ
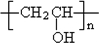 ��
����2��C�й����ŵ�������̼̼˫����ȩ����A��F�к˴Ź������׳���������C��D����� ����ţ���
��3����Ӧ�۵Ļ�ѧ����ʽΪCH3COOH+CH��CH��CH3COOCH=CH2����Ӧ�����Ǽӳɷ�Ӧ����Ӧ�ܵĻ�ѧ����ʽΪ
 ��
����4��PVAc����F�Ӿ۶��ɣ�д��������F������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ��HCOOCH=CHCH3��HCOOCH2CH=CH2��CH3OOCCH=CH2��HCOOC��CH3��=CH2���������֣���
���� AΪ����һԪ������ͨʽΪCnH2n+2O����������������ԼΪ34.8%������$\frac{16}{12n+2n+2+16}$��100%=34.8%������n=2������AΪCH3CH2OH���������и�����ת����ϵ��A������EΪCH3COOH��E����Ȳ�����ӳɷ�Ӧ��FΪCH3COOCH=CH2��F�����Ӿ۷�Ӧ��PVAc������ˮ���PVAΪ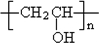 ��A��ͭ��������������������BΪCH3CHO��B������Ϣ���еķ�Ӧ��CΪCH3CH=CHCHO��C������ԭ��Ӧ��DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB���ݴ˴��⣮
��A��ͭ��������������������BΪCH3CHO��B������Ϣ���еķ�Ӧ��CΪCH3CH=CHCHO��C������ԭ��Ӧ��DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB���ݴ˴��⣮
��� �⣺AΪ����һԪ������ͨʽΪCnH2n+2O����������������ԼΪ34.8%������$\frac{16}{12n+2n+2+16}$��100%=34.8%������n=2������AΪCH3CH2OH���������и�����ת����ϵ��A������EΪCH3COOH��E����Ȳ�����ӳɷ�Ӧ��FΪCH3COOCH=CH2��F�����Ӿ۷�Ӧ��PVAc������ˮ���PVAΪ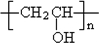 ��A��ͭ��������������������BΪCH3CHO��B������Ϣ���еķ�Ӧ��CΪCH3CH=CHCHO��C������ԭ��Ӧ��DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB��
��A��ͭ��������������������BΪCH3CHO��B������Ϣ���еķ�Ӧ��CΪCH3CH=CHCHO��C������ԭ��Ӧ��DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB��
��1����������ķ�����֪��A�Ļ�ѧ����Ϊ�Ҵ���PVA�Ľṹ��ʽΪ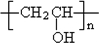 ��
��
�ʴ�Ϊ���Ҵ���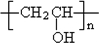 �� ��������
�� ��������
��2��CΪCH3CH=CHCHO��C�й����ŵ�������̼̼˫����ȩ����A��F�к˴Ź������׳���������C��D������4�ַ壬
�ʴ�Ϊ��̼̼˫����ȩ����C��D��
��3����Ӧ�۵Ļ�ѧ����ʽΪCH3COOH+CH��CH��CH3COOCH=CH2����Ӧ�����Ǽӳɷ�Ӧ����Ӧ�ܵĻ�ѧ����ʽΪ  ��
��
�ʴ�Ϊ��CH3COOH+CH��CH��CH3COOCH=CH2���ӳɷ�Ӧ�� ��
��
��4��FΪCH3COOCH=CH2����F������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪ��HCOOCH=CHCH3��HCOOCH2CH=CH2��CH3OOCCH=CH2��HCOOC��CH3��=CH2��
�ʴ�Ϊ��HCOOCH=CHCH3��HCOOCH2CH=CH2��CH3OOCCH=CH2��HCOOC��CH3��=CH2���������֣���
���� ���⿼���л���ĺϳɣ�Ϊ�߿��������ͣ��ۺϿ���ѧ�������������ۺ����û�ѧ֪ʶ����������Ŀ�ѶȽϴ����ʱע��������е���Ϣ��ע������л�������ŵĽṹ�����ʣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | �ǽ�����Cl��Br���ʽ�Cl2ͨ��NaBr��Һ�У�������ӦΪ��Cl2+2Br-=Br2+2Cl- | |
| B�� | �ǽ�����F��Br�������ԣ�HF��HBr | |
| C�� | �ǽ�����S��33As����ǰ�ߵ���̬�⻯���ȶ��Ը�ǿ | |
| D�� | �ǽ�����O��N����O2��H2���ϱ�N2������ |
| A�� | ����������Ư�۾�����Ҫ�ɷ� | |
| B�� | �����ƿ����ں������ | |
| C�� | ���������Ӽ���ʳ������彡�����к�������ʳ�� | |
| D�� | ��������������Դ����������β����������������߿������� |
 �����Ϊ1L���ܱ������У�������䣩����1mol CO2��3mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g�� ���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ������˵����ȷ���ǣ�������
�����Ϊ1L���ܱ������У�������䣩����1mol CO2��3mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g�� ���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ���е�3����ʱ������Ӧ���ʺ��淴Ӧ������� | |
| B�� | 10���Ӻ������и�����Ũ�Ȳ��ٸı� | |
| C�� | �ﵽƽ��������¶ȣ�����Ӧ���������淴Ӧ���ʼ�С | |
| D�� | 3minǰv����v����3min��v����v�� |
ʵ��һ����Na2SO4��Һ�еμ�AgNO3��Һ
| ��� | Na2S04��Һ | AgN03��Һ | ���� | ||
| ���/mL | Ũ��/��mol•L-1�� | ���/�� | Ũ��/��mol•L-1�� | ||
| �� | 1 | l | 3 | 2 | ���ִ�����ɫ���� |
| �� | 1 | 1 | 3 | 0.5 | ����������ɫ���� |
| �� | 1 | 1 | 3 | 0.1 | �������� |
| �� | 1 | 1 | 3 | 0.0l | �����Ա仯 |
��2��ѧ����������±���ʵ��һ���ݽ������ۼ��㣬�������ɱ������в�Ҫ���ո�
[25��ʱKsp��Ag2SO4��=1.2��10-5��Ksp��AgCl��=1.8��10-10]
| ��� | AgNO3Ũ��/��mol•L-1�� | ϡ�ͺ�Ag+Ũ��/��mol•L-1�� | ���Һ��SO42-����С���ۼ��Ũ��/��mol•L-1�� |
| �� | 2 | 0.2 | 0.0003 |
| �� | 0.5 | 0.0048 | |
| �� | 0.1 | 0.0l | 0.12 |
| �� | 0.001 |
A�����Һ��c��SO42-��=0.1mol/Lʱ�������Ag2SO4����
B�����Һ��c��SO42-��=1mol/Lʱ�������Ag2SO4����
c������SO42-Ũ�ȴ�С�������Ag2SO4����
D����ʹ��0.01mol/L AgNO3��Һ���ɻ����ų�SO42-��Cl-���鹹�ɵĸ���
��3����ʵ��һ�б�Ţ��е����ۼ�������������գ����������ϴ�Ag+Ӧ���γɳ��������롰��Щ�����ǡ���������ì�ܣ�Ϊ̽�����࣬��ʵ��һ�Ļ����ϼ������������ʵ�飮
ʵ�����
| ��� | AgNO3��Һ Ũ��/��mol•L-1�� | ���� | ������еμ����������� |
| �� | 2 | ���ִ�����ɫ���� | �μ�ϡ���ᣬ���������ܽ⣻�ļ�Ũ���ᣬ�����Ͽ���ʧ |
| �� | 0.5 | ����������ɫ���� | �μ�ϡ���ᣬ����������ʧ |
����һ��H+��Ag2SO4�ܽ������ã�
�������NO3-��Ag2SO4�ܽ������ã�
��4����������ѡ�Լ���ѡ���ʵ��Լ������ʵ�鷽�����ֱ���֤����һ�ͼ�����Ƿ�������� д��ʵ�鲽��ͽ��ۣ�����ѡ�Լ���Ag2SO4���塢ŨHNO3��NaNO3������Һ��CaSO4���壩��ȡ����CaSO4�������Թ��У�����һ����ŨHNO3��������������ܽ⣬˵������һ������
��ȡ����Ag2SO4�������Թ��У�����һ����NaNO3������Һ��������������ܽ⣬˵�����������
��5��ͨ����4����ʵ�飬��֤ʵ����һ����������ƽ�����۽���Ag2SO4�ܽ��ԭ��Ag2SO4������ˮ�д���ƽ�⣺Ag2SO4��s��?2Ag+��aq��+SO42-��aq����H+��SO42-�������HSO4-��SO42-Ũ�Ƚ��ͣ�ƽ�������ƶ���Ag2SO4�����ܽ⣮
| A�� | HCl��ǿ���H2SO3������ | B�� | HClO4�����Ա�H2SO4ǿ | ||
| C�� | H2S��HCl�ȶ� | D�� | H2SO4��HClO����ǿ |
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | �� | |||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� |
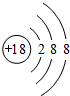 ��
����2���ؿ��к������Ľ���Ԫ����Al��д������ܰ�1��1�γɵĻ�����ĵ���ʽ
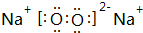 �����к��еĻ�ѧ�������Ӽ������ۼ���
�����к��еĻ�ѧ�������Ӽ������ۼ�����3����ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ����HClO4��������ǿ����KOH��
��4��д���ۡ��ߡ����Ӧ�ļ������ӵĻ�ԭ����ǿ�����Ĵ���S2-��Cl-��F-
��5���ܵ�����������ˮ����͢ݵ�������������Ӧ�����ӷ���ʽ��Al2O3+2OH-=2AlO2-+H2O
��6���ݺ͢���ɵĻ��������й��ۼ�����ѧ�����ͣ���
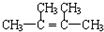 ��
��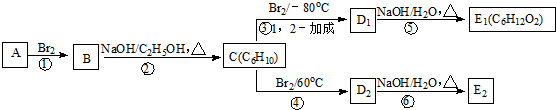
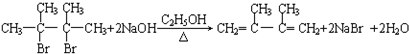 ��
��