��Ŀ����
10����ͼ��Ԫ�����ڱ���һ���֣����еĢ١�����Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش�| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | �� | |||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� |
 ��
����2���ؿ��к������Ľ���Ԫ����Al��д������ܰ�1��1�γɵĻ�����ĵ���ʽ
 �����к��еĻ�ѧ�������Ӽ������ۼ���
�����к��еĻ�ѧ�������Ӽ������ۼ�����3����ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ����HClO4��������ǿ����KOH��
��4��д���ۡ��ߡ����Ӧ�ļ������ӵĻ�ԭ����ǿ�����Ĵ���S2-��Cl-��F-
��5���ܵ�����������ˮ����͢ݵ�������������Ӧ�����ӷ���ʽ��Al2O3+2OH-=2AlO2-+H2O
��6���ݺ͢���ɵĻ��������й��ۼ�����ѧ�����ͣ���
���� ����Ԫ�������ڱ���λ�ã���֪��ΪN����ΪO����ΪF����ΪNa����ΪAl����ΪSi����ΪS����ΪCl����ΪAr����ΪK��
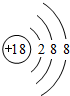
��1��Arԭ�������Ϊ�ȶ��ṹ����ѧ��������ã�ԭ�Ӻ�����18�����ӣ���3�����Ӳ㣬���������Ϊ2��8��8��
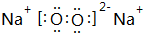
��2���ؿ��к������Ľ���Ԫ����Al������ܰ�1��1�γɵĻ�����ΪNa2O2��
��3������������Ӧ��ˮ�����У�������ǿ���Ǹ����ᣬK�Ľ�������ǿ��������ǿ�����������أ�
��4���ǽ�����Խǿ�������ӵĻ�ԭ��Խ����
��5���ܵ�����������ˮ����ΪNaOH���ݵ����������Ϊ�����������߷�Ӧ����ƫ��������ˮ��
��6���ݺ͢���ɵĻ�����ΪAlCl3��
��� �⣺����Ԫ�������ڱ���λ�ã���֪��ΪN����ΪO����ΪF����ΪNa����ΪAl����ΪSi����ΪS����ΪCl����ΪAr����ΪK��
��1��Arԭ�������Ϊ�ȶ��ṹ����ѧ��������ã�ԭ�Ӻ�����18�����ӣ���3�����Ӳ㣬���������Ϊ2��8��8��ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2���ؿ��к������Ľ���Ԫ����Al������ܰ�1��1�γɵĻ�����ΪNa2O2��������������������ӹ��ɣ�����ʽΪ ���������Ӽ������ۼ���
���������Ӽ������ۼ���
�ʴ�Ϊ��Al�� �����Ӽ������ۼ���
�����Ӽ������ۼ���
��3������������Ӧ��ˮ�����У�������ǿ���Ǹ����ᣬ��ѧʽΪHClO4��K�Ľ�������ǿ��������ǿ����KOH���ʴ�Ϊ��HClO4��KOH��
��4���ǽ�����F��Cl��S���ǽ�����Խǿ�������ӵĻ�ԭ��Խ���������ӻ�ԭ�ԣ�S2-��Cl-��F-���ʴ�Ϊ��S2-��Cl-��F-��
��5���ܵ�����������ˮ����ΪNaOH���ݵ����������Ϊ�����������߷�Ӧ����ƫ��������ˮ����Ӧ���ӷ���ʽΪ��Al2O3+2OH-=2AlO2-+H2O���ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��6���ݺ͢���ɵĻ�����ΪAlCl3�����й��ۼ����ʴ�Ϊ�����ۼ���
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ��ؼ���������Ԫ�����ڱ��Ľṹ�ƶ�Ԫ�أ������ڻ���֪ʶ�Ĺ��̣�

| A�� | Ũ�����Ǹ߷е���ᣬͨ������Ũ���ᷴӦ��ȡ�ͷе���� | |
| B�� | ͨ���ı��¶Ⱥ�Ũ�ȵ�����������ƽ���ƶ�ԭ����ȡHCl | |
| C�� | ����ǿ���ϣ��ܽ�Ȼ��Ӱ�죬���ܽ�ȵ��������� | |
| D�� | Ũ�����Ũ�ȣ�98%��Զ����Ũ�����Ũ�ȣ�37%������Ũ�ȵ�����ȡ��Ũ�ȵ��� |
| A�� |
| B�� |
| C�� |
| D�� |
|
�ٵ����� ����������ľ̿���� �۾ƾ�����ˮ ��HCl��������ˮ
�ݱ��ڻ� ��NH4Cl�������������������ۻ� �ࣨNH4��2SO4����ˮ��
| A�� | �٢ܢޢ� | B�� | �ܢޢ� | C�� | �٢ڢܢ� | D�� | �ܢ� |
| A�� | �������������������ˮ�����ŷſ�ʹˮ�帻Ӫ���� | |
| B�� | ���������ŷ�SO2��ȫ�������ů����Ҫԭ�� | |
| C�� | ��չ��̼���á�ѭ�����ã��ƹ�����̫���ܡ����ܵij�������ϵͳ | |
| D�� | �Ͼɵ���еĹ����ӡ�Ǧ���ؽ�����������ˮԴ�������Ⱦ |

| A�� | ��ع���ʱ��������ӦʽΪ��O2+2 H2O+4e-=4OH- | |
| B�� | ���ʱ��a �缫��Χ���ȷŵ����Cl- | |
| C�� | ���ʱ����������·���ǣ����������·����������Һ������������ | |
| D�� | ����������ģ������������0.2g H2ʱ��b����Χ�����2.24LH2 |
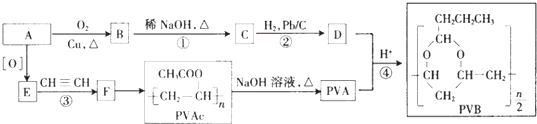
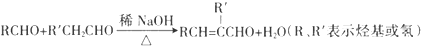
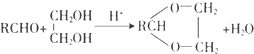
 ��
��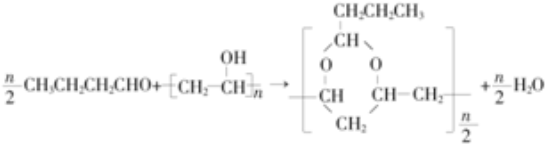 ��
�� E��
E�� ��
��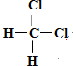
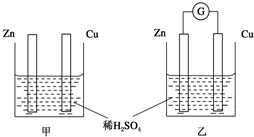 �ֱ���ͼ�ס�����ʾװ�ý���ʵ�飬ͼ�������ձ������ҺΪͬŨ�ȵ�ϡ���ᣬ����GΪ�����ƣ���ش��������⣺
�ֱ���ͼ�ס�����ʾװ�ý���ʵ�飬ͼ�������ձ������ҺΪͬŨ�ȵ�ϡ���ᣬ����GΪ�����ƣ���ش��������⣺