��Ŀ����
10��Ϊ�ⶨij±��������ɣ�ijͬѧ���ʵ�����£�����ȡ��±����Һ��11.4mL����������NaOH��Һ�����ȷ�Ӧ��Һ�岻�ֲ㣻����ȴ����ϡ�����ữ���ټ���������������۹��˳�����ϴ�ӳ���2��3�Σ��ܽ������������أ��ش��������⣺
��1�����м���NaOH��Ŀ�����к�±����ˮ���������±�ᣬʹˮ����ȫ
��2�����м��������������Ŀ����ʹ±������ȫ����
��3�����������ɵij���Ϊ����ɫ�����±�����е�±ԭ����Br
��4�����Ƶó���������Ϊ37.6g���ֲ��±�������ܶ�Ϊ1.65g•mL-1���������ܶ�����ͬ�����������ܶȵ�94�������±���������к���2��±ԭ�ӣ�
��5��д����±�������ܵĽṹ��ʽ��CH2BrCH2Br��CH3CHBr2��
���� ��1��±�����ڼ�����������ȫˮ�⣻
��2����������������ʹ±��������ȫ������
��3�����������ɵij���Ϊ����ɫ����ó���ΪAgBr��
��4������n=$\frac{m}{M}$����AgBr�����ʵ��������л���������ܶ�����ͬ�����������ܶȵ�94�����������Է�������������m=��V������л�����������ٸ���n=$\frac{m}{M}$���±���������ʵ�����ȷ��±�����е�±ԭ����Ŀ��
��5������Br��ԭ������±��������Է����������±������������ʽ����������ʽ������12����Ϊ̼ԭ����������Ϊ��ԭ�������ݴ�д������ʽ�ͽṹ��ʽ��
��� �⣺��1��±�����ڼ�����������ȫˮ�⣬���Լ���NaOH��Ŀ�����к�±����ˮ�������±���⣬ʹˮ����ȫ���ʴ�Ϊ���к�±����ˮ�������±���⣬ʹˮ����ȫ��
��2��������������ʹ±�����ӳ�����ȫ�����Ը������ó�������ɫ��������ȷ��±��ԭ�ӵ�����������ʴ�Ϊ��ʹ±������ȫ������
��3�����������ɵij���Ϊ����ɫ����ó���ΪAgBr�����Ը�±�����е�±ԭ����Br���ʴ�Ϊ��Br��
��4�����Ƶó���������Ϊ37.6g��n��AgBr��=$\frac{m}{M}$=$\frac{37.6g}{188g/mol}$=0.2mol��
��֪���л���������ܶ�����ͬ�����������ܶȵ�94����������Է�������Ϊ188��m=��V=1.65g•mL-1��11.4mL=18.8g�����л�������ʵ���n=$\frac{m}{M}$=$\frac{18.8g}{188g/mol}$=0.1mol��Br�����ʵ�����±���������ʵ���֮��Ϊ2��1������±�����е�Brԭ����ĿΪ2��
�ʴ�Ϊ��2��
��5����֪±�����е�Brԭ����ĿΪ2������Է�������Ϊ188�����Է�����������ʽ��Ϊ188-80��2=28��������ʽ������12����Ϊ̼ԭ����������Ϊ��ԭ���������Ը�����Ϊ-C2H4������±�����ķ���ʽΪC2H4Br2��������ṹ��ʽΪ��CH2BrCH2Br��CH3CHBr2��
�ʴ�Ϊ��CH2BrCH2Br��CH3CHBr2��
���� ���⿼����±��������ʽ��ȷ����������Ŀ�ѶȽϴ�ע��������ȷ��������������ʽ������12����Ϊ̼ԭ����������Ϊ��ԭ������

| A�� | CH2Br2 | B�� | C3H7Cl | C�� | C4H10 | D�� | C |

| A�� | ��Ag������O2��Ӧʱ���١��ۼ����� | B�� | ���Ӽ���ˮʱ���٢ڼ����� | ||
| C�� | ��Ũ���Ṳ����170��ʱ���ڡ��ܼ����� | D�� | ����ᡢŨ���Ṳ��ʱ���ڼ����� |
| A�� | 63.47 | B�� | 64.47 | C�� | 63.57 | D�� | 64.57 |
| A�� | X2+�ĺ��������ĿΪ18����X�ڵ������ڵڢ�A�� | |
| B�� | Mg��OH��2���Ա�Ca��OH��2ǿ | |
| C�� | Ԫ�����ڱ���7�����壬7�����壬1��0�壬1�����壬��16���� | |
| D�� | Li������ý�����F������÷ǽ��� |
| A�� | ����������������ͭ�������� | B�� | �١��ڡ��۾���������Ʒ�Ӧ | ||
| C�� | �١��ڡ��۾��ܷ���ȡ����Ӧ | D�� | һ�������£��ܿ���ת��Ϊ�� |
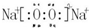 ��
��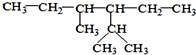 ��ϵͳ����Ϊ��2��4-����-3-�һ����飮
��ϵͳ����Ϊ��2��4-����-3-�һ����飮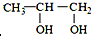 ��
�� 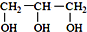 ���������������=������ͬ��
���������������=������ͬ�� ��
��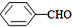
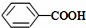 ��CH3COOH����ʾ����ȷ��봼�����ԣ�
��CH3COOH����ʾ����ȷ��봼�����ԣ�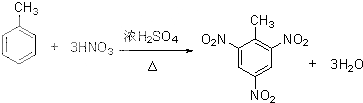 ��
��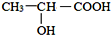 ���ڴ��������£��ϳɾ����ᣨPLA���ķ�Ӧ����ʽ��
���ڴ��������£��ϳɾ����ᣨPLA���ķ�Ӧ����ʽ�� ��
��