��Ŀ����
1��������ѧ���ش��������⣺��1����
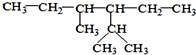 ��ϵͳ����Ϊ��2��4-����-3-�һ����飮
��ϵͳ����Ϊ��2��4-����-3-�һ����飮��3-��-2-�����Ľṹ��ʽ��CH3CH��OH��CH��CH3��2��
���Ҷ����׳Ʋ��ᣮ
��2�������Ŷ��л���������������ã���Ҳ���ܵ��������ŵ�Ӱ�죮
�ٱȽϷе�
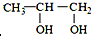 ��
�� 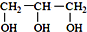 ���������������=������ͬ��
���������������=������ͬ���ڱȽ�ˮ���ԣ�
 ��
��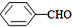
�۱Ƚ����ԣ�
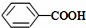 ��CH3COOH����ʾ����ȷ��봼�����ԣ�
��CH3COOH����ʾ����ȷ��봼�����ԣ���3���ϳ��л����Ѿ��������������еõ��㷺��Ӧ�ã�
����д���ױ��ϳ�TNT�ķ�Ӧ����ʽ��
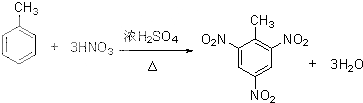 ��
������д�����ᣨ
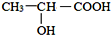 ���ڴ��������£��ϳɾ����ᣨPLA���ķ�Ӧ����ʽ��
���ڴ��������£��ϳɾ����ᣨPLA���ķ�Ӧ����ʽ�� ��
��
���� ��1���� ���̼��Ϊ6���ֱ���2��4��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����3-��-2-�����Ľṹ��ʽΪ��CH3CH��OH��CH��CH3��2�����Ҷ����׳Ʋ��
���̼��Ϊ6���ֱ���2��4��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����3-��-2-�����Ľṹ��ʽΪ��CH3CH��OH��CH��CH3��2�����Ҷ����׳Ʋ��
��2����̼ԭ����Խ�࣬�ǻ���Խ�࣬�е�Խ�ߣ�
��ȩ��ˮ������Ȼ��ܣ�����������ˮ��������ȩ�����ܽ�ȣ�
�۱��������Ȼ��ϵ������ӵ�����γɵĸ����Ӻͱ����Ĺ���ṹ�������
��3���ٰ�ŨH2SO4��ŨHNO3�ͼױ���ϼ����Ʊ�TNT���DZ�������ԭ�ӱ�����ȡ�������������ױ�������ԭ���غ���ƽ��ѧ����ʽ��
������ͨ��������Ӧ���е����۷�Ӧ���ɾ����ᣮ
��� �⣺��1���� ���̼��Ϊ6���ֱ���2��4��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����ȷ����Ϊ��2��4����-3-�һ����飬�ʴ�Ϊ��2��4-����-3-�һ����飻
���̼��Ϊ6���ֱ���2��4��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����ȷ����Ϊ��2��4����-3-�һ����飬�ʴ�Ϊ��2��4-����-3-�һ����飻
��3-��-2-�����Ľṹ��ʽΪ��CH3CH��OH��CH��CH3��2���ʴ�Ϊ��CH3CH��OH��CH��CH3��2��
���Ҷ���ͨ���Զ�ˮ�������ʽ���ڣ��׳Ʋ��ᾧ�壬�ʴ�Ϊ�����
��2����̼ԭ����Խ�࣬�ǻ���Խ�࣬�е�Խ�ߣ��������к���3��C��3���ǻ��� �к���3��C��2���ǻ����ʱ������ķе����
�к���3��C��2���ǻ����ʱ������ķе���� ���ʴ�Ϊ������
���ʴ�Ϊ������
��ȩ��ˮ������Ȼ��ܣ�����������ˮ��������ȩ�����ܽ�ȣ�����ˮ���ԣ� ��
��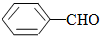 ���ʴ�Ϊ����������
���ʴ�Ϊ����������
�۱��������Ȼ��ϵ������ӵ�����γɵĸ����Ӻͱ����Ĺ���ṹ�������ʹ����������ӱ���������Ӹ��ȶ����������Ա�����ǿ���ʴ�Ϊ������
��3���ٰ�ŨH2SO4��ŨHNO3�ͼױ���ϼ����Ʊ�TNT�Ļ�ѧ����ʽΪ��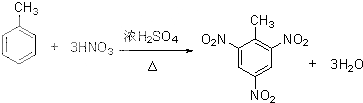 ���ʴ�Ϊ��
���ʴ�Ϊ��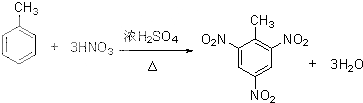 ��
��
������ͨ��������Ӧ���е����۷�Ӧ���ɾ����ᣬ��Ӧ����ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ������Ҫ��������л��������������ṹ��ʽ����д���л����۷е��Լ�����ǿ���ıȽϣ��л���ѧ��Ӧ����ʽ����д�ȣ��ۺ��Խ�ǿ����һ�����Ѷȣ�

| A�� | H2XO3 | B�� | H2XO4 | C�� | HXO3 | D�� | HXO4 |
 Ϊ̽��FeCl2��Һ���ʱ�������IJ��ij��ȤС��������װ�ý���ʵ�飺���������ϣ�����ܷ������缫��Ӧ�������ʣ����ѹ��С������Ũ�ȵ������йأ���
Ϊ̽��FeCl2��Һ���ʱ�������IJ��ij��ȤС��������װ�ý���ʵ�飺���������ϣ�����ܷ������缫��Ӧ�������ʣ����ѹ��С������Ũ�ȵ������йأ�����缫a����ʼ��δ�������ݣ�������������ɫ�н�������Ĺ��壮�õ缫�ĵ缫��ӦʽΪ��Fe2++2e-=Fe��
��缫b��ʼһ��ʱ���ڣ����������ݲ�������ΧҺ����ֺ��ɫ�����ǣ���ȡ�õ缫��Χ����Һ������֧�Թ��У�һ֧�Թ��е������-KI��Һ��Һ����ɫ���䣻��һ֧�Թ����ȼ������ữ���ٵ���KSCN��Һ����Һ��Ϊ��ɫ��
���ۣ��������в�����Fe3+��ͬʱˮ������˺��ɫ���ʣ�
��1���������ữʱ�����ķ�Ӧ��Fe��OH��3+3H+=Fe3++3H2O�������ӷ���ʽ��ʾ����
��2���Ե�������Fe3+������ԭ����в��룺
����٣�Cl-�������ŵ磬���ɵ�Cl2��Fe2+������Fe3+��
����ڣ�Fe2+������ֱ�ӷŵ�����Fe3+��
����ۣ�����
��3�����ʵ����֤�����
��ѡ����Լ���1mol/L FeSO4��Һ��1mol/L���ᡢ2mol/L���ᡢ1mol/L NaCl��Һ��2mol/L NaCl��Һ������-KI��Һ��KSCN��Һ������ˮ
| ���� | ����ͽ��� |
| ��ȡһ����2mol/LNaCl��Һ��������ҺpHΪ4.91��������ͬװ�ý��е�⣮ �ڵ����ͬʱ������缫b���������ݣ�ȡ����������������Һ�� �������KI��Һ�� | ����Һ��������֤������ٲ����� ����Һ������֤������ٿ��ܳ����� |
| A�� | 0.1 mol•L-1 NH4Cl��Һ��c��NH4+����c�� Cl-�� | |
| B�� | ���������Һ�м����������ᣬ�õ������Ի����Һ��c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| C�� | 0.1 mol•L-1 Na2CO3��Һ��c��Na+��=2c��HCO3-��+2c��CO32-��+2c��H2CO3�� | |
| D�� | NaHCO3��Һ�У�c��H+��+c��Na+��=c��OH-��+2c��CO32-��+c��HCO3-�� |
������ԭ��ص������ǣ�Cu���缫��Ӧʽ��2H++2e-=H2����
�������������Һ��Ϊ����ͭ��Һ�������ǣ�Zn�������缫��Ӧʽ��Zn-2e-=Zn2+�������缫��Ӧʽ��Cu2++2e-�TCu������������ͬ�ĵ���ʱ�����������������Ϊ12.9g��
��2����A��B��C��D���ֽ������±���װ�ý���ʵ�飮
| װ�� |  |  |  |
| ���� | ���۽���A�����ܽ� | C���������� | A����������� |
��װ�ü��и����ĵ缫��Ӧʽ�ǣ�A-2e-�TA2+��
��װ�����������ĵ缫��Ӧʽ�ǣ�Cu2++2e-�TCu��
��װ�ñ�����Һ��pH�����������С�����䡱����
�����ֽ��������ǿ������˳����D��A��B��C��
| A�� | 2A��l��+B��l��=2C��g����H1=-Q1 | B�� | 2A��g��+B��g��=2C��g����H2=-Q2 | ||
| C�� | 2A��g��+B��g��=2C��l����H3=-Q3 | D�� | 2A��l��+B��l��=2C��l����H4=-Q4 |
| �¶ȣ� T �� | K1 | K2 |
| 973 | 1.47 | 2.38 |
| 1173 | 2.15 | 1.67 |
��2�����з�Ӧ��H2��g��+CO2��g��?CO��g��+H2O��g����H=Q3ƽ�ⳣ��ΪK3
a�����ݷ�Ӧ������Ƶ���K1��K2��K3�Ĺ�ϵʽK3=$\frac{{K}_{1}}{{K}_{2}}$�����ƶϷ�Ӧ����������š����������ȷ�Ӧ��Ҫʹ��Ӧ����һ�������½�����ƽ�����ƣ��ɲ�ȡ�Ĵ�ʩ��BD��
A����С������� B�������¶� C��ʹ�ú��ʵĴ��� D���跨����CO���� E�������¶�
b�����ݷ�Ӧ������Ƶ���Q1��Q2��Q3�Ĺ�ϵʽQ3=Q1-Q2��
| A�� | CH3COOCH3 | B�� | HCHO | C�� | HCOOH | D�� | CH3OH |