��Ŀ����
10��д�����з�Ӧ���Ȼ�ѧ����ʽ��1����25�棬101kPa ��1mol �Ҵ���C2H5OH����ȫȼ�ղ����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ1366.8kJ����д����ʾ�Ҵ�ȼ�յ��Ȼ�ѧ����ʽ��C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��l����H=-1366.8 kJ•mol-1
��2���£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬��ȼ���Ƚϴ���ȼ�ղ���Ի�������Ⱦ���ʿ����������ȼ�ϣ�
��֪��N2��g��+2O2��g��=2NO2��g����H=+67.7kJ/mol
2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g����H=һ1135.7kJ/mol[��
������ȫȼ�յ��Ȼ�ѧ����ʽΪN2H4��l��+O2��g��=N2��g��+2H2O��l����H=-534kJ/mol��
���� ��1������ȼ���ȵĶ����𣬱�ʾȼ���ȵ��Ȼ�ѧ����ʽ�п�ȼ��Ϊ1mol������Ϊ�ȶ����������1mol �Ҵ���C2H5OH����ȫȼ�ղ����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ1366.8kJ��д���Ȼ�ѧ����ʽ��
��2�������Ȼ�ѧ����ʽ��˹���ɼ���õ�����+�ڣ���$\frac{1}{2}$�õ�N2H4��l��+O2��g��=N2��g��+2H2O��l�����ݴ˼����ʱ䣮
��� �⣺��1��ȼ������ָ����25�桢101KPaʱ��1mol��������ȫȼ�������ȶ���������ų���������
1mol �Ҵ���C2H5OH����ȫȼ�ղ����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ1366.8kJ����ȼ���ȵ��Ȼ�ѧ����ʽΪ��C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��l����H=-1366.8 kJ•mol-1��
�ʴ�Ϊ��C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��l����H=-1366.8 kJ•mol-1��
��2����N2��g��+2O2��g��=2NO2��g����H=+67.7kJ/mol
��2N2H4��l��+2NO2��g��=3N2��g��+4H2O��l����H=-1135.7kJ/mol
��ϸ�˹���ɼ��㣨��+�ڣ���$\frac{1}{2}$�õ�N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-534kJ/mol��
�ʴ�Ϊ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-534kJ/mol��
���� ���⿼����ȼ���ȵĸ����Լ�ȼ���ȵ��Ȼ�ѧ����ʽ����д�Լ���˹���ɵ�Ӧ�ã�ע��ȼ���ȵ��Ȼ�ѧ����ʽ�п�ȼ������ʵ���Ϊ1mol������������ȶ����������Ŀ�ϼ�

| A�� | 2Q1=Q | B�� | 2Q1��Q | C�� | 2Q1��Q | D�� | ���ж� |
| A�� | ����40.0 L N2����״���� | |
| B�� | ��0.250 mol KN03������ | |
| C�� | ת�Ƶ��ӵ����ʵ���Ϊ1.25 mol | |
| D�� | ��������Nԭ�ӵ����ʵ���Ϊ2.5mol |
| A�� | 16O��18O��Ϊͬλ�أ�H2${\;}_{\;}^{16}$O��D2${\;}_{\;}^{16}$O��H2${\;}_{\;}^{18}$O��D2${\;}_{\;}^{18}$O��Ϊͬ�������� | |
| B�� | SiH4��PH3��HCl���ȶ�������ǿ | |
| C�� | �������Ļ�ѧʽ��FeO | |
| D�� | Ca2+�Ľṹʾ��ͼΪ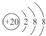 ��NH4Cl�ĵ���ʽΪ ��NH4Cl�ĵ���ʽΪ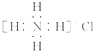 |
| A�� | CaCO3���̣� | B�� | NaCl��Һ | C�� | KNO3��Һ | D�� | CuSO4���̣� |
| X | Y | Z | M | R | Q | |
| ԭ�Ӱ뾶/nm | 0.186 | 0.074 | 0.099 | 0.143 | ||
| ��Ҫ���ϼ� | -4��+4 | -2 | -1��+7 | +3 | ||
| ���� | �����Ӻ������� | ���ǽ������ϵ����� | ��ɫ��Ӧ�ʻ�ɫ |
��2��Z�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ��2Na+2H2O=2NaOH+H2����
��3��Y��R��ȣ�R�ǽ����Խ�ǿ����ԭ�ӽṹ�ǶȽ���ԭ��ͬ����������ң�ԭ�Ӱ뾶��С���õ���������ǿ���ǽ�������ǿ����ǽ�����Cl��Si����ʵ����ʵ�ǶȽ��ͣ�������ʵ��֤����һ���۵���bc��ѡ����ĸ��ţ���
a��������Y�ĵ��ʳʹ�̬��R�ĵ��ʳ���̬
b���ȶ���XR��YX4
c��Y��R�γɵĻ�������Y������
d����XRˮ��Һ����Z2YM3��Һ��������
��4�����ݱ��������Ʋ⣬Y��ԭ�Ӱ뾶����Сȡֵ��Χ�Ǵ���0.099nmС��0.143nm��
| A�� | ��CΪ���壬��Bһ�������� | |
| B�� | B��Cһ���������� | |
| C�� | ����ʼʱ��������Ͷ��18gA����Ӧ���յ�����Ϊ44.5kJ | |
| D�� | ����ʼʱ��������Ͷ��18gA����Ӧ�ų�������Ϊ44.5kJ |
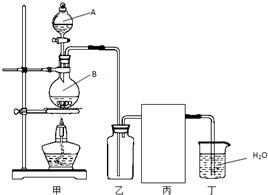 ��1��ʵ�����Ʊ�HCl����ķ�ӦΪ��
��1��ʵ�����Ʊ�HCl����ķ�ӦΪ��