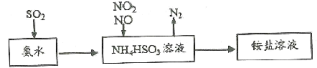
��Ŀ����
����Ŀ��ʵ������H2O2�����������ڴ��������Ȼ�林����£�ѡ�����̿��Ϊ�����Ʊ����Ȼ���������(III)�����(���ԵĶ����ܰ����������Ϊ���Ե������ܰ������)���������£�

��֪��Co(NH3)6Cl3�ڲ�ͬ�¶���ˮ�е��ܽ��������ͼ��

(һ)���Ȼ���������(III)�������Ʊ�
(1)�������Ҫ��ȴ��10���ٻ����ر߽������H2O2��Һ������������Ŀ���ǣ�________��
(2)ʵ�����Ʊ����Ȼ���������(III)�ܷ�Ӧ��ѧ����ʽΪ��_______________________��
(3)ʵ�����1Ϊ______________������2��[Co(NH3)6]Cl3��Һ�м���Ũ�����Ŀ����____________________________________________��
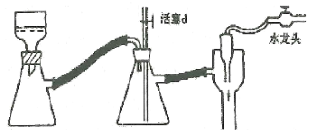
(4)ʵ�����õ���ѹ����װ����ͼ����������ϻ���;ֹͣ����ʱ��Ӧ����ȡ����ȷ����������_________________��
(��)��Ʒ��NH3�����IJⶨ
�ֳ�ȡ0.1000g��Ʒ����������ƿ�з������·�Ӧ��
[Co(NH3)x]Cl3+3NaOH=Co(OH)3��+xNH3��+3NaCl(����ͼ)����ƿ��װ��10.00mL c mol��L-1 H2SO4��������ƿ��ʹNH3��ȫ�ݳ����μ�2��ָʾ������0.5000mol��L-1 NaOH����Һ�ζ����ζ��ﵽ�յ�ʱ����NaOH��ҺV mL��
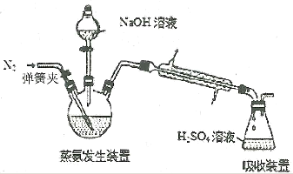
(5)���й���ʵ���˵������ȷ����______________��
A.�ڢٲ�����NH4Cl��Һ�м�����ϸ��CoCl2��6H2O���壬Ŀ���Ǽ��ٹ�����ܽ�
B.ԭ��NH4Cl����Ҫ����������NH3��H2O�ĵ��룬���������ɶ����ܰ��������γ�Cu(OH)2
C.��ѹ�����漰ת����Һ�������ǣ�����������ת����Һ����ˮ��ͷ������Һ������ʱ��ת�Ƴ���
D.�ڢܲ���ʹ�ú�������ķ�ˮ���ٽ���Ʒ������
E.�ζ�ʱ�����2��ָʾ���Ƿ�̪
(6)����ʵ��(��)�����ݼ��㣺������NH3����������Ϊ__________(�ú���ĸ��ʽ�ӱ�ʾ)��
���𰸡���ֹ�¶ȹ���H2O2�ֽ⣬NH3��H2O�ֽ⣻���ͷ�Ӧ���ʣ���ֹ��Ӧ���ھ��� 2CoCl2��6H2O+10NH3+2NH4Cl+H2O2 2[Co(NH3)6]Cl3+14H2O ���ȹ��� ������[Co(NH3)6]Cl3�����������ͬ����ЧӦ������߲��� �ȴ���d����ر�ˮ��ͷ DE (0.34c-0.085V) ��100%
2[Co(NH3)6]Cl3+14H2O ���ȹ��� ������[Co(NH3)6]Cl3�����������ͬ����ЧӦ������߲��� �ȴ���d����ر�ˮ��ͷ DE (0.34c-0.085V) ��100%
��������
��1����Ӧ����H2O2����ˮ�����������ʶ����Ȳ��ȶ���ʹ��ʱ��Ҫע�ⷴӦ�����⣻���⣬����������Ϊ�˷�ֹ��Ӧ���ھ��ң�
��2���������е���Ϣ���÷�Ӧ�ķ�Ӧ����CoCl2��6H2O��NH3��NH4Cl��H2O2����������[Co(NH3)6]Cl3��H2O2�Ļ�ԭ����һ����H2O���ݴ�д����ѧ����ʽ����ƽ���ɣ�
��3����[Co(NH3)6]Cl3�ͻ���̿�Ļ����Һ�м��뺬ŨHCl��ˮ��������I�õ�[Co(NH3)6]Cl3��Һ�������IΪ���ȹ��ˣ���[Co(NH3)6]Cl3��Һ�м���ŨHCl�����Դٽ�[Co(NH3)6]Cl3�����������ͬ����ЧӦ����
��4����������ϻ���;ֹͣ����ʱ��Ӧ����ȡ����ȷ�����������ȴ���d����ر�ˮ��ͷ��
��5��A��������������뵽��Һ�У�Ŀ���Ǽӿ��ܽ⣻
B����ˮ�д���:NH3+H2O![]() NH3��H2O
NH3��H2O![]() NH4++OH-������ƽ���ƶ���ԭ��������
NH4++OH-������ƽ���ƶ���ԭ��������
C����ʵ�������ȷ��
D�����뺬HCl�ķ�ˮ�������˵õ�[Co(NH3)6]Cl3��Һ����Ȼ�ò�������Ϊ���������壻
E���ζ��ﵽ�յ�ʱ����Һ����NH4+�������ԣ�����ʹ�ü�����ָʾ������
��6�����ݵζ����㼴�ɡ�
��1����Ӧ����H2O2����ˮ�����������ʶ����Ȳ��ȶ���ʹ��ʱ����������Ҫ���£���ֹ���������ʷֽ⣻���⣬����������Ϊ�˷�ֹ��Ӧ���ھ��ң�
��2���÷�Ӧ�Ļ�ѧ����ʽΪ��2CoCl2��6H2O+10NH3+2NH4Cl+H2O2 2[Co(NH3)6]Cl3+14H2O��
2[Co(NH3)6]Cl3+14H2O��
��3����[Co(NH3)6]Cl3�ͻ���̿�Ļ����Һ�м��뺬ŨHCl��ˮ��������I�õ�[Co(NH3)6]Cl3��Һ�������IΪ���ȹ��ˣ���[Co(NH3)6]Cl3��Һ�м���ŨHCl�����Դٽ�[Co(NH3)6]Cl3�������������߲��ʣ�
��4����������ϻ���;ֹͣ����ʱ��Ӧ����ȡ����ȷ�����������ȴ���d����ر�ˮ��ͷ��
��5��A��ȷ��
B����ˮ�д���:NH3+H2O![]() NH3��H2O
NH3��H2O![]() NH4++OH-������Һ�м���NH4Cl����������NH3��H2O�ĵ��룬��СOH-��Ũ�ȣ�����NH3��Ũ�ȣ��Դٽ������ܰ����������ɣ�����Cu(OH)2�����ɣ�B��ȷ��
NH4++OH-������Һ�м���NH4Cl����������NH3��H2O�ĵ��룬��СOH-��Ũ�ȣ�����NH3��Ũ�ȣ��Դٽ������ܰ����������ɣ�����Cu(OH)2�����ɣ�B��ȷ��
C��ȷ��
D�����뺬HCl�ķ�ˮ�������˵õ�[Co(NH3)6]Cl3��Һ����ò��費��Ϊ�˴ٽ���Ʒ��������D����
E���ζ��ﵽ�յ�ʱ����Һ����NH4+�������ԣ�����ʹ�ü�����ָʾ�����������÷�̪��E����
��ѡDE��
��6��n(NaOH)=V��10-3L��0.5mol/L=0.5V��10-3mol������2NaOH-H2SO4����ʣ��H2SO4 0.25V��10-3mol��������NH3��H2SO4��(0.01c-0.25V��10-3)mol������2NH3-H2SO4�������յ�NH3��(0.02c-0.5V��10-3)mol![]() ��100%=(0.34c-0.085V) ��100%��
��100%=(0.34c-0.085V) ��100%��

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�����Ŀ������ͼװ���У���ƿ�г�����������a������ͷ�ι��е�Һ��b������ƿ�ڣ���������ƿ��Ȼ����ɼ�f���ձ��е�Һ��b����Ȫ״��������ռ���������ƿ��a��b�ֱ������
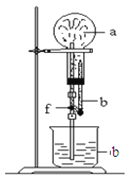
��������a | Һ��b | |
A | NO2 | ˮ |
B | C12 | ����ʳ��ˮ |
C | NH3 | ˮ |
D | CO2 | 4 mol��L��1NaOH��Һ |
A.AB.BC.CD.D