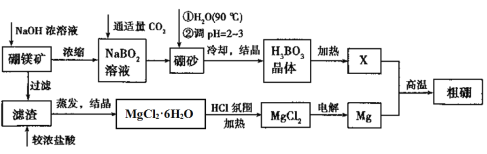
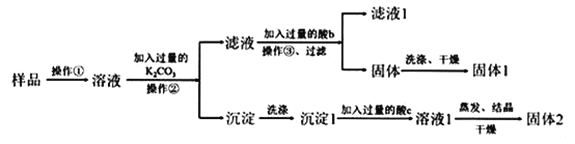
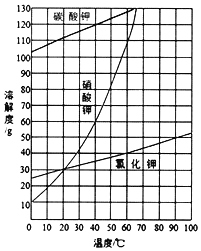
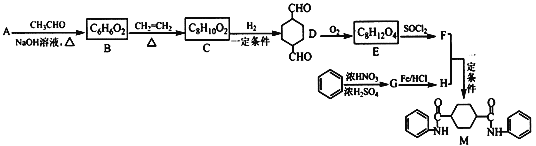
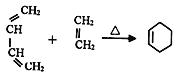
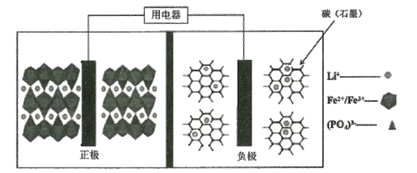
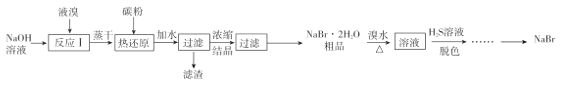
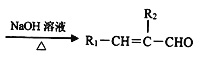��Ŀ����
����Ŀ��ij��ɫ��Һ�п��ܺ���Na����K����NH4+��Ca2����Cu2����SO42-��SO32-��Cl����Br����CO32-�е������֣�����Ũ�ȶ�Ϊ0.1 mol��L��1��������Һ�м��������BaCl2������Ļ����Һ���ް�ɫ�������ɡ�ijͬѧ��ȡ����ԭ��Һ����Ʋ��������ʵ�飺�����й���ԭ��Һ���ж���ȷ���ǣ� ��

A.��������Ba(NO3)2��HNO3�Ļ����Һ����BaCl2������Ļ����Һ�������Һ�����ӵ��ж���Ӱ��
B.��ȷ��ԭ��Һ���Ƿ����Cl��
C.�϶����ڵ�������SO32-��Br�����Ƿ����Na����K����Ҫͨ����ɫ��Ӧ��ȷ��
D.�϶������ڵ�������Ca2����Cu2����SO42-��CO32-���Ƿ�NH4+����ʵ����֤
���𰸡�A
��������
��ɫ��Һ��һ������Cu2+,������Һ�м��������BaCl2������Ļ����Һ,�ް�ɫ��������,˵����![]() ,��������ˮ,���������,����
,��������ˮ,���������,����![]() ,��Һ�����Ȼ�̼��Һ,�²�Ȼ�ɫ,����Br-,�ϲ�����ᱵ��ϡ�����а�ɫ��������,����
,��Һ�����Ȼ�̼��Һ,�²�Ȼ�ɫ,����Br-,�ϲ�����ᱵ��ϡ�����а�ɫ��������,����![]() ,��Mg2+����Һ������Ũ�ȶ�Ϊ0.1 mol��L-1,���ݵ���غ�,һ������
,��Mg2+����Һ������Ũ�ȶ�Ϊ0.1 mol��L-1,���ݵ���غ�,һ������![]() ��Na+��K+,һ��������Cl-����Һ�м������ữ���������а�ɫ��������,����Ϊ����ˮʱ�����������ӡ�
��Na+��K+,һ��������Cl-����Һ�м������ữ���������а�ɫ��������,����Ϊ����ˮʱ�����������ӡ�
A. ע������Ƿ�����������ӣ��������ᱵ����������ص�����жϣ���Һ���Ѻ��������ӣ�����û�й�ϵ����A�������⣻
B. ������Ŀ��Ũ�ȶ�������õ���غ�����жϳ��Ƿ��������ӣ���B ����
C. �϶����ڵ�������![]() ��Br-��
��Br-��![]() ��Na+��K+,��C����
��Na+��K+,��C����
D. ���ݵ���غ�����жϳ���Һ����笠����ӣ���D����
����ȷ��Ϊ��A��

 53���ò�ϵ�д�
53���ò�ϵ�д�����Ŀ��ijС�����������װ�ý���ʵ�飬�ڡ�������Һ���������������������±���
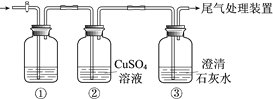
ʵ�� | ���� | ���� |
�� | ��ʢ��Na2S��Һ�Ģ��г���ͨ��CO2������ | ���в�����ɫ��������Һ��pH���ͣ� ���в�����ɫ���ǣ��û�������ð���� |
�� | ��ʢ��NaHCO3��Һ�Ģ��г���ͨ��H2S���������� | ����ͬʵ��� |
���ϣ�CaS��ˮ��ȫˮ��
������ʵ��ó��Ľ�������ȷ����
A. ���а�ɫ������CaCO3
B. ������ҺpH���͵�ԭ���ǣ�H2S+Cu2+ == CuS��+2H+
C. ʵ������CO2���������ķ�Ӧ�ǣ�CO2+H2O+ S2== CO32+ H2S
D. ��ʵ���͢��ܱȽ�H2CO3��H2S���Ե�ǿ��