��Ŀ����
6����Ҫ������������⣺��1����������Һ��ͨ�������̼�ķ���ʽ��C6H5O-+CO2+H2O��C6H5OH+NHCO3-��
��2����ȩ����������Ӧ�Ļ�ѧ����ʽ��CH3CHO+2Ag��NH3��2OH$\stackrel{��}{��}$CH3COONH4+H2O+2Ag��+3NH3��
��3������Ũ���ᡢŨ�����Ϻ������50�桫60�淢����Ӧ�ķ���ʽ��
 ��
����4���ƾ۱���ϩ�Ļ�ѧ����ʽ��
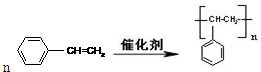 ��
����5�����ᣨ2-�ǻ����ᣩ��һ�����������۵Ļ�ѧ����ʽ��
 ��
��
���� ��1���������������̼��Ӧ���ɱ��Ӻ�̼�����ƣ�
��2����ȩ�к��й�����ȩ�����ܹ���������Һ����������Ӧ��������李������ʡ�ˮ�Ͱ�����
��3������Ũ���ᡢŨ�����ͺ������50�桫60�淢��ȡ����Ӧ��������������ˮ��
��4������ϩ�к�̼̼˫���������Ӿ۷�Ӧ���ɾ۱���ϩ��
��5��2-�ǻ������к����Ȼ����ǻ����������۷�Ӧ���� ��
��
��� �⣺��1�����ӵ����Դ���̼��������ӣ����߷�Ӧ���ɱ��Ӻ�̼�����ƣ���Ӧ�Ļ�ѧ����ʽΪ��C6H5ONa+CO2+H2O��C6H5OH+NaHCO3�����ӷ�ӦΪ��C6H5O-+CO2+H2O��C6H5OH+NHCO3-��
�ʴ�Ϊ��C6H5O-+CO2+H2O��C6H5OH+NHCO3-��
��2����ȩ��������Һ��Ӧ�����������ʺ����ᣬ��Ӧ�ķ���ʽΪ��CH3CHO+2Ag��NH3��2OH$\stackrel{��}{��}$CH3COONH4+H2O+2Ag��+3NH3��
�ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH$\stackrel{��}{��}$CH3COONH4+H2O+2Ag��+3NH3��
��3������Ũ���ᡢŨ�����ͺ������50�桫60�淢��ȡ����Ӧ��������������ˮ���÷�ӦΪ ��
��
�ʴ�Ϊ�� ��
��
��4��һ�������£�����ϩ�����ۺϷ�Ӧ���ɾ۱���ϩ����Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��5��2-�ǻ������к����Ȼ����ǻ����������۷�Ӧ����Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���˳����л���Ӧ����ʽ����д����Ŀ�Ѷ��еȣ�ע�����ճ������л���Ӧԭ�����ܹ���ȷ��д��Ӧ�Ļ�ѧ����ʽ������������ѧ�����Ӧ�û���֪ʶ��������

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�| A�� | �к͵�����������ʵ���Ũ������ʹ�����Һ����������NaOH��Һ���ڴ��� | |
| B�� | �����£�20 LpH=12��Na2CO3��Һ�к��е�OH-������Ϊ0.2NA | |
| C�� | ��0.1 mol/LCH3COOH��Һ�м�������CH3COONa�� �壬��Һ��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ ���� | |
| D�� | һ���¶��£�10mL 0.50mol•L-1NH4Cl��Һ��20mL 0.25mol•L-1NH4C1��Һ��NH4+���ʵ�����ͬ |
| A�� | $\frac{3}{8}$ mol | B�� | $\frac{8}{3}$ mol | C�� | $\frac{2}{3}$ mol | D�� | $\frac{3}{2}$ mol |
| A | ||
| B | C | D |
��2��Ԫ��A�ڻ�������һ���Ը����������ۣ�������F����Ԫ�ط��ţ��γɻ�����ʱ������ʾ�ļ�̬�����෴��
��3���Ƚ�C��DԪ�ؼ����Ӱ뾶�Ĵ�СS2-��Cl-�������ӷ��ţ���
��4����D��E�γɵĻ������ˮ��Һ�м��������ˮ���йط�Ӧ�����ӷ���ʽΪMg2++2 NH3��H2O�TMg ��OH��2��+2 NH4+��
��5��D������������Ӧ��ˮ������E������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪMg��OH��2+2HClO4�TMg��ClO4��2+2H2O��
| A�� | ����������Һ�з����û���Ӧ | |
| B�� | 1 mol���������ڷ�Ӧ��ʧȥ���ӵĶ��� | |
| C�� | ����Ԫ�ص�����������ˮ����ļ���ǿ�� | |
| D�� | ����������ˮ�����û������������� |

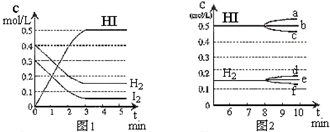 ��ijһ�ݻ�Ϊ2L���ܱ������ڣ�����0.8mol��H2��0.6mol��I2����һ���������·������·�Ӧ��H2��g��+I2��g��?2HI��g����H��0����Ӧ�и����ʵ�Ũ����ʱ��仯�����ͼ1��
��ijһ�ݻ�Ϊ2L���ܱ������ڣ�����0.8mol��H2��0.6mol��I2����һ���������·������·�Ӧ��H2��g��+I2��g��?2HI��g����H��0����Ӧ�и����ʵ�Ũ����ʱ��仯�����ͼ1��