��Ŀ����
��15�֣��������ʹ���������������õ���Ҫ�о�����
��1��������A(H3BNH3)��һ��DZ�ڵĴ�����ϣ�������Ԫ��״����(HB��NH)3ͨ�����·�Ӧ�Ƶã�3CH4��2(HB��NH)3��6H2O��3CO2��6H3BNH3����ش�
��H3BNH3���Ƿ������λ�� ����ǡ�����B��C��N��O��һ�������ɴ�С��˳��Ϊ ��CH4��H2O��CO2�����Ӱ��ռ����ɴ�С��˳������Ϊ ��
����(HB��NH)3��Ϊ�ȵ�����ķ���Ϊ �������ʽ��
���˹����Ժϳ����һϵ���⻯��������������������ƣ��ʳ�֮Ϊ���顣��ҵ�Ͽɲ���LiAlH4��BCl3��һ���������Ʊ�������B2H6���÷�Ӧ�Ļ�ѧ����ʽΪ ��
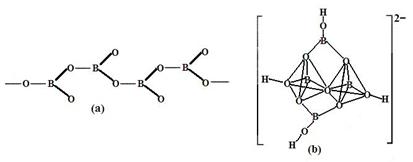
�����������У�����������״����״���Ǽ�״�ȶ��ֽṹ��ʽ��ͼ��Ϊһ��������״�ṹ�Ķ���������仯ѧʽΪ ��ͼ��Ϊ��ɰ�����������ӣ�������ԭ�Ӳ�ȡ���ӻ���ʽΪ ��
��2��һ��ͭ�Ͻ���д����
��Cu2+�ļ۲�����Ų�ʽΪ ��
��ͭ����������������仯���ﶼ���Է�����ɫ��Ӧ����ԭ���� ��
��ͭ�ĵ����а�ABCABC������ʽ�ѻ�����ͭԭ�Ӱ뾶Ϊa pm����þ�����ܶ�Ϊ g/cm3������٤������ֵΪNA��
��1��������A(H3BNH3)��һ��DZ�ڵĴ�����ϣ�������Ԫ��״����(HB��NH)3ͨ�����·�Ӧ�Ƶã�3CH4��2(HB��NH)3��6H2O��3CO2��6H3BNH3����ش�
��H3BNH3���Ƿ������λ�� ����ǡ�����B��C��N��O��һ�������ɴ�С��˳��Ϊ ��CH4��H2O��CO2�����Ӱ��ռ����ɴ�С��˳������Ϊ ��
����(HB��NH)3��Ϊ�ȵ�����ķ���Ϊ �������ʽ��
���˹����Ժϳ����һϵ���⻯��������������������ƣ��ʳ�֮Ϊ���顣��ҵ�Ͽɲ���LiAlH4��BCl3��һ���������Ʊ�������B2H6���÷�Ӧ�Ļ�ѧ����ʽΪ ��
�����������У�����������״����״���Ǽ�״�ȶ��ֽṹ��ʽ��ͼ��Ϊһ��������״�ṹ�Ķ���������仯ѧʽΪ ��ͼ��Ϊ��ɰ�����������ӣ�������ԭ�Ӳ�ȡ���ӻ���ʽΪ ��

��2��һ��ͭ�Ͻ���д����
��Cu2+�ļ۲�����Ų�ʽΪ ��
��ͭ����������������仯���ﶼ���Է�����ɫ��Ӧ����ԭ���� ��
��ͭ�ĵ����а�ABCABC������ʽ�ѻ�����ͭԭ�Ӱ뾶Ϊa pm����þ�����ܶ�Ϊ g/cm3������٤������ֵΪNA��
��1�����ǣ�N>O>C>B CO2>CH4>H2O;��C6H6 ����4BCl3+3LiAlH4=2B2H6+3LiCl+3AlCl3
����4BCl3+3LiAlH4=2B2H6+3LiAlCl4����BO2--��sp3��sp2����2����3d9���ڼ���̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ����һ�������Ĺ����ʽ�ͷ��������� ��
��
����4BCl3+3LiAlH4=2B2H6+3LiAlCl4����BO2--��sp3��sp2����2����3d9���ڼ���̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ����һ�������Ĺ����ʽ�ͷ���������
 ��
�������������1������H3BNH3�е�B��Nԭ��֮�������λ����B��C��N��O��ͬһ���ڵ�Ԫ�ء�һ������£�Ԫ�صķǽ�����Խǿ��ԭ�Ӱ뾶ԽС��Ԫ�صĵ�һ�����ܼ���Խ��������Nԭ�ӵ�2p����ϵĵ��Ӵ��ڰ�������ȶ�״̬������������ܱ�O������˵�һ�������ɴ�С��˳��N>O>C>B��CH4����������ṹ������109��28�䣻H2O��V�ͷ��ӣ�����104.3�㣬CO2��ֱ���ͷ��ӣ�����Ϊ180�㡣����������Ӱ��ռ����ɴ�С��˳������ΪCO2>CH4>H2O���ڵȵ�������ԭ�����ȣ�����������Ҳ��ȵ����ʡ���(HB��NH)3��Ϊ�ȵ�����ķ���ΪC6H6 ���۸�������ɵúϳ�������B2H6���Ļ�ѧ����ʽΪ4BCl3+3LiAlH4=2B2H6+3LiCl+3AlCl3��Ҳ��д��4BCl3+3LiAlH4=2B2H6+3LiAlCl4���ܶ�������Ļ�ѧʽΪBO2--����ͼ����ԭ�Ӳ�ȡ���ӻ���ʽΪsp3��sp2����2����Cu��29��Ԫ�أ�Cu2+�ļ۲�����Ų�ʽΪ3d9����ͭ����������������仯���ﶼ���Է�����ɫ��Ӧ��ԭ����������ʱԭ���еĵ��������������ӻ�̬ԾǨ������̬�����Ǽ���̬�Dz��ȶ��ģ�����̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ����һ�������Ĺ����ʽ�ͷ���������Cu�������������ܶѻ�����һ�������к��е�Cuԭ�ӵĸ���Ϊ��8��1/8+6��1/2="4;" ��ͭԭ�Ӱ뾶Ϊa pm.�����ı߳�L.
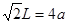 ;L=2
;L=2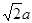 ;�þ�����ܶ�Ϊ
;�þ�����ܶ�Ϊ g/cm3.
g/cm3.
��ϰ��ϵ�д�
�����Ŀ

 g��cm��3�������ı߳�Ϊacm�����ӵ�����Ϊ__ mol-l���ú��ѡ�a��ʽ�ӱ�ʾ����
g��cm��3�������ı߳�Ϊacm�����ӵ�����Ϊ__ mol-l���ú��ѡ�a��ʽ�ӱ�ʾ����
