��Ŀ����
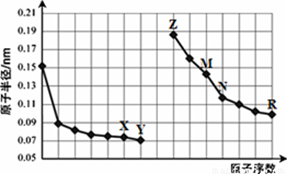
���ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ����±���
����˵����ȷ���ǣ� ��
A��Ԫ��X��Q�γɵĻ������в����ܺ��й��ۼ�
B��X��Z��R������������ˮ����֮����������Ӧ
C��R3-��Q2-������ʧȥ����
D��M(OH)2�ļ��Ա�XOH�ļ���ǿ
| Ԫ�ش��� | X | Y | Z | M | R | Q | |
| ԭ�Ӱ뾶(��10-10 m) | 1.86 | 0.99 | 1.43 | 1.60 | 0.75 | 0.74 | |
| ��Ҫ���ϼ� | ������� | +1 | +7 | +3 | +2 | +5 | ���� |
| ����� | ���� | ��1 | ���� | ���� | -3 | -2 | |
A��Ԫ��X��Q�γɵĻ������в����ܺ��й��ۼ�
B��X��Z��R������������ˮ����֮����������Ӧ
C��R3-��Q2-������ʧȥ����
D��M(OH)2�ļ��Ա�XOH�ļ���ǿ
BC
������������ݱ����и�Ԫ��ԭ�ӵİ뾶��Ԫ�صĻ��ϼۿ����ƶϳ������е�Ԫ���ǵ�2��3����Ԫ�أ�����X��Y��Z��M�ֱ���Ԫ��Na��Cl��Al��Mg����R��Q����Ԫ��N��O����˸����Ƶ�Ԫ�أ�����Ԫ�����ڱ���Ԫ�������ɽ��з�����A��X��Q�����γɹ������ƣ����й��ۼ���A����B��X��Z��R������������ˮ����ֱ����������ơ��������������ᣬ��˿����������Ӧ��B��ȷ��N�ǽ����Ա�O��������N3-��O2-������ʧȥ������ȷ��������Naǿ��Mg������Mg (OH)2�ļ��Ա�NaOH�ļ�������D����ѡBC��

��ϰ��ϵ�д�
�����Ŀ