��Ŀ����
�ɼ��ֳ������λ����ɵİ�ɫ��ĩ������ֻ���ܺ���Na����K����Al3����CO32����HCO3����SO42����NO2���е����������ӡ�ijͬѧ�Ը���Һ��������ʵ�飺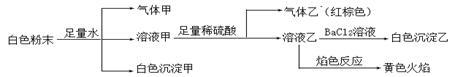
����˵����ȷ����
| A����ɫ��������Al(OH)3���������CO2�����Ի����һ����Al3����HCO3�� |
| B��������һ����NO��NO2�Ļ������ |
| C����ɫ��������BaSO4������ԭ�����һ������SO42�� |
| D����ɫ��ĩ��һ������Na�� ��Al3�� ��NO2�� |
D
���������������Ϊ�ڰ�ɫ��ĩ�м���������ˮ����������塢��ɫ������˵�������ܹ�˫ˮ����������һ������Al3��������˫ˮ���������HCO3������CO32-����ȷ�����������CO2����������Al(OH)3�������ڰ�ɫ��ĩ�м���������в���NaԪ�أ�����Һ�ҵ���ɫ��ӦΪ��ɫ��˵����ԭ������Һ�к���Na��������Һ���м���������ϡ��������������Ǻ���ɫ��˵��ԭ��Һ�к���NO2�����ӡ����ڼ�����ϡ���ᣬ�������ԭ������Һ���Ƿ���SO42�����ӣ���Һ����һ������SO42���������Һ�м���BaCl2��Һʱ�����BaSO4�����������������û����һ�����е�������Na�� ��Al3�� ��NO2����ѡ��ΪD��
���㣺�������ӵļ������ε�ˮ���֪ʶ��

 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵���( )
| ѡ�� | �� | �� | �� | ʵ����� |  |
| A | ϡ���� | Na2S | AgNO3��AgCl����Һ | Ksp(AgCl)��Ksp(Ag2S) | |
| B | Ũ���� | ���� | ��ˮ | Ũ���������ˮ�ԡ������� | |
| C | ϡ���� | Na2SO3 | Ba(NO3)2��Һ | SO2������Ա��ξ��������ɰ�ɫ���� | |
| D | Ũ���� | Na2CO3 | Na2SiO3��Һ | ���ԣ����̼����� |
����˵����ȷ����
| A��0.1 mol/L��Һ�е�(NH4)2SO4��Һ�е�c(NH4��)<c(SO42��) |
| B����ͬ�¶��£�0.6 mol/L��ˮ��Һ��0.3 mol/L��ˮ��Һ��c(OH��)֮����2��1 |
| C����0.1 mol/L NaNO3��Һ�еμ�����ʹ��ҺpH=5����ʱ���Һ�е�c(Na��)<c(NO3��) |
| D�����������Һ�м����������ᣬʹ���ҺpH=7����ʱ���Һ��c(Na��)<c(CH3COO��) |
����������ȷ����
| A��0��1mol��L��1CH3COONa��Һ�У�c��Na������c��CH3COO������c��H������c��OH���� |
| B��NH4Cl��Һ��ˮϡ�ͺָ���ԭ�¶ȣ�pH��KW������ |
| C��pH��4��CH3COOH��Һ��pH��4��NH4Cl��Һ�У�c��H��������� |
| D����NaHCO3��Һ�У�c��OH������c��CO32-����c��H������c��H2CO3�� |
��25��ʱ����50��00 mLδ֪Ũ�ȵ�CH3COOH��Һ����μ���0.5 mol��L-1��NaOH��Һ���ζ������У���Һ��pH�����NaOH��Һ����Ĺ�ϵ��ͼ��ʾ��������˵���У���ȷ����
| A�����к͵ζ����̣�������ʯ����ָʾ�� |
B��ͼ�е����ʾ��Һ�У� |
| C��ͼ�е����ʾ��Һ��ˮ�ĵ���̶ȴ��ڵ����ʾ��Һ��ˮ�ĵ���̶� |
D���ζ������е�ij�㣬���� �Ĺ�ϵ���� �Ĺ�ϵ���� |
��֪������AgCl��Ksp��1.8��10��10��AgI��Ksp��8.5��10��17������5mL����KCl��KI��Ϊ0.01mol/L�Ļ����Һ�м���8mL 0.01mol/L AgNO3��Һ����ʱ��Һ���������ʵ�����Ũ�ȴ�С��ϵ��ȷ����
| A��c��K+����c��NO3������c��Cl������c��Ag+����c��I���� |
| B��c��K+����c��NO3������c��Ag+����c��Cl������c��I���� |
| C��c��NO3������c��K+����c��Ag+����c��Cl������c��I���� |
| D��c��K+����c��NO3������c��Ag+����c��Cl������c��I���� |
����Һ�������25�棬�й���������ȷ����
| A��pH��4.5�ķ���֭��c(H��)��pH��6.5��ţ����c(H��)��100�� |
| B��ij���ʵ���Һ����ˮ�����c(H��)=1��l0-12mol��L��1���������һ����ǿ����Һ |
| C��AgCl��ͬŨ�ȵ�CaCl2��NaCl��Һ�е��ܽ�Ȳ���ͬ |
| D��pH��5.6��CH3COOH��CH3COONa�����Һ�У�c(Na��) �� c(CH3COO��) |
 NaOH��Һ����μ��롣0.2mol
NaOH��Һ����μ��롣0.2mol



