��Ŀ����
����˵����ȷ����
| A��0.1 mol/L��Һ�е�(NH4)2SO4��Һ�е�c(NH4��)<c(SO42��) |
| B����ͬ�¶��£�0.6 mol/L��ˮ��Һ��0.3 mol/L��ˮ��Һ��c(OH��)֮����2��1 |
| C����0.1 mol/L NaNO3��Һ�еμ�����ʹ��ҺpH=5����ʱ���Һ�е�c(Na��)<c(NO3��) |
| D�����������Һ�м����������ᣬʹ���ҺpH=7����ʱ���Һ��c(Na��)<c(CH3COO��) |
C
�������������A������笠�����ˮ�⣬��(NH4)2SO4��Һ�е�c(NH4��)>c(SO42��)������B������������ʶ��ԣ���ҺԽϡԽ���룬����0.3 mol/L��ˮ��Һ��һˮ�ϰ��ĵ���̶ȴ����Զ��ߵ�c(OH��)֮��С��2:1������C����������Һ�����ԣ��μ��������Һ�����ԣ�������Һ��c(Na��)<c(NO3��)����ȷ��D�������Һ�����ԣ���c��OH����= c(H��)�����ݵ���غ���Һ��c(Na��)=c(CH3COO��)������ѡC��
���㣺������Һ������Ũ�ȵıȽ�

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�����Һ���ճ������б��㷺Ӧ�á�ij����Һ��ɫ��pH>7����ijЩ��ɫ������Ư�����á�����Ϊ�����ܵ���Ч�ɷ���
| A��NaCl | B��Na2CO3 | C��KMnO4 | D��NaClO |
������Һ�и����ӵ�Ũ�ȹ�ϵ����ȷ����(����)
| A��0��1 mol��L��1CH3COONa��Һ�У�c(CH3COO��)��c(CH3COOH)��0��1 mol��L��1 |
| B��Ũ�Ⱦ�Ϊ0��1 mol��L��1 Na2CO3��Һ��ϡ����������Ϻ����Һ�У� c(CO 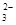 )��c(OH��)��c(H��)��c(H2CO3) )��c(OH��)��c(H��)��c(H2CO3) |
| C��25 ��ʱ��pH��9��4��Ũ�Ⱦ�Ϊ0��1 mol��L��1��HCN��NaCN�Ļ����Һ�У� c(Na��>c(CN��)>c(HCN)>c(OH��) |
| D�� 3��0 L 0��1 mol��L��1 NaOH��Һ�л���ͨ��CO2����Һ����8��8 gʱ����Һ�У� |
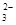 )>c(HCO
)>c(HCO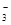 )>c(OH��)>c(H��)
)>c(OH��)>c(H��) ��֪ij�¶���CH3COOH��NH3?H2O �ĵ��볣����ȣ�����10mLŨ��Ϊ0.1mol?L?1��CH3COOH��Һ�еμ���ͬŨ�ȵİ�ˮ���ڵμӹ�����
| A��ˮ�ĵ���̶�ʼ������ |
| B��c��NH4+��/c��NH3?H2O���������ټ�С |
| C��c��CH3COOH����c��CH3COO?��֮��ʼ�ձ��ֲ��� |
| D�������백ˮ�����Ϊ10mLʱ��c(NH4+)=c(CH3COO?) |
�Գ�����pH=3��CH3COOH��Һ��������������ȷ����
A�� |
| B����������CH3COONa�����c��CH3COO�������� |
| C������Һ����ˮ�������c��H+����1.0��10-11 mol��L |
| D��������pH =11��NaOH��Һ��Ϻ�������Һ������ |
�ɼ��ֳ������λ����ɵİ�ɫ��ĩ������ֻ���ܺ���Na����K����Al3����CO32����HCO3����SO42����NO2���е����������ӡ�ijͬѧ�Ը���Һ��������ʵ�飺
����˵����ȷ����
| A����ɫ��������Al(OH)3���������CO2�����Ի����һ����Al3����HCO3�� |
| B��������һ����NO��NO2�Ļ������ |
| C����ɫ��������BaSO4������ԭ�����һ������SO42�� |
| D����ɫ��ĩ��һ������Na�� ��Al3�� ��NO2�� |
�����£���20.00 mL 0.100 mol��L��1CH3COONa��Һ����μ���0.100 mol��L��1���ᣬ��Һ��pH����������������Ĺ�ϵ��ͼ��ʾ(�����ǻӷ�)������˵����ȷ����
| A�������ʾ��Һ�У�c(Na��)��c(Cl��)��c(H��)��c(OH��) |
| B�������ʾ��Һ�У�c(Na��)��c(Cl��)��c(CH3COO��)��c(CH3COOH) |
| C�������ʾ��Һ�У�c(Na��)��c(CH3COOH)��c(H��)��c(CH3COO��) |
| D�����������п��ܳ��֣�c(H��)+c(Na��)��c(CH3COO��)+c(CH3COOH) |
�����£�0.1mol/L�İ�ˮpH��11�����������������
| A�������Һ�м�ˮϡ�ͣ�c(OH��)/c(NH3��H2O )���� |
| B��0.lmol/L��ˮ��0.lmol/LH2SO4��Һ�������Ϻ�������Һ�У�c(NH4+)+c(H+)��2c(SO42��)+c(OH��) |
| C��0.1mol/L��ˮ��0.05mol/LHCl��Һ�������Ϻ�������Һ�У�c(NH4+)+n(NH3)+n(NH3��H2O)��2n(Cl��) |
| D��Ũ�Ⱦ�Ϊ0.1mol/L��ˮ��NH4Cl��Һ�������Ϻ�����Һ�ʼ��ԣ��� |