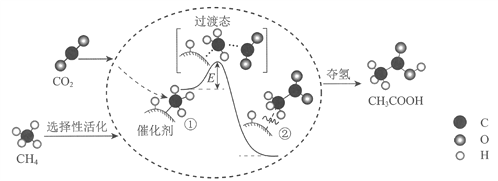
��Ŀ����
����Ŀ��һ�������£������Ϊ3 L���ܱ������У�һ����̼��������Ӧ���ɼ״���
������ΪCu2O/ZnO����CO(g)��2H2(g)![]() CH3OH(g)���״������ʵ������¶ȵĹ�ϵ����ͼ��ʾ�����з�����ȷ���� �� ��
CH3OH(g)���״������ʵ������¶ȵĹ�ϵ����ͼ��ʾ�����з�����ȷ���� �� ��

A. ��Ӧ�ﵽƽ��ʱ��ƽ�ⳣ������ʽ K��c(CH3OH)/c(CO)��c2(H2)
B. �����¶ȣ�Kֵ����
C. ��500�����ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)��3tB/2nBmol��(L��min)��1
D. �����������䣬E������ѹ����ԭ����1/2������ƽ��ʱn(H2)/n(CH3OH)����
���𰸡�A
��������
A��ƽ�ⳣ��Ϊ��������Ũ�ȵĻ�ѧ���������ݵij˻������Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻��ı�ֵ����Ӧ�ﵽƽ��ʱ��ƽ�ⳣ������ʽΪK��c(CH3OH)/c(CO)��c2(H2)��ѡ��A��ȷ��
B����ͼ���֪B�����ȵõ�ƽ�⣬�¶ȸ�ƽ��ʱ�״������ʵ��������ͣ�˵������Ӧ�Ƿ��ȷ�Ӧ���������¶�ƽ�����淴Ӧ�����ƶ���Kֵ��С��ѡ��B����ȷ��
C���¶�Ϊ500��ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״������ʵ���ΪnBmol����ʱ�״���Ũ��Ϊ![]() mol/L���������ɼ״���ƽ������Ϊ��v(CH3OH)=
mol/L���������ɼ״���ƽ������Ϊ��v(CH3OH)=![]() mol��L-1��min-1����Ϊ��Ӧ����֮������Ӧ�Ļ�ѧ������֮�ȣ����������ķ�Ӧ������
mol��L-1��min-1����Ϊ��Ӧ����֮������Ӧ�Ļ�ѧ������֮�ȣ����������ķ�Ӧ������![]() mol��L-1��min-1��ѡ��C����ȷ��
mol��L-1��min-1��ѡ��C����ȷ��
D���������������������£�������E�����ϵ���ѹ����ԭ����1/2��ƽ��������Ӧ������У���������Ũ����Ȼ�����ӵģ�ѡ��D����ȷ��
��ѡA��

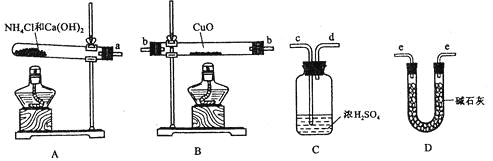
����Ŀ��ij�ռ���Ʒ�����������������õ�����,Ϊ�˲ⶨ�䴿��,�������µζ�������
A. ��250 mL������ƿ�ж�������250 mL�ռ���Һ��
B. �ü�ʽ�ζ�����ȡ25.00 mL�ռ���Һ����ƿ�в�����2�μ���ָʾ����
C. ����ƽ��ȷ��ȡ�ռ���Ʒ2.0 g,���ձ���������ˮ�ܽ⣻
D. �����ʵ���Ũ��Ϊ0.100 0 mol��L��1�ı�����װ����ʽ�ζ���,����Һ����¿�ʼ����ΪV1��
E. ����ƿ�µ�һ�Ű�ֽ,�ζ����յ�,���¶���V2��
�ʹ�ʵ�����������գ�
��1����ȷ�IJ��������˳����(�ñ����ĸ��д)
________��________��________��D��________��
��2������E����ƿ�µ�һ�Ű�ֽ��������_______________________________
��3���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��______________��ֱ������һ���������Һ____________________________ (����ɫ�仯)��
��4�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵�����____________��
A.��ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B.�ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C.��ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D.��ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
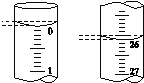
��5�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ��������������Һ�����Ϊ________mL��
��6��ijѧ������3��ʵ��ֱ��¼�й����������
�ζ� ���� | ����NaOH��Һ�����/mL | 0.100 0 mol��L��1��������/mL | ||
�ζ�ǰ �̶� | �ζ���̶� | ��Һ���/mL | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
�����ϱ�������ʽ������ռ�Ĵ���____�������������λ��Ч���֣�