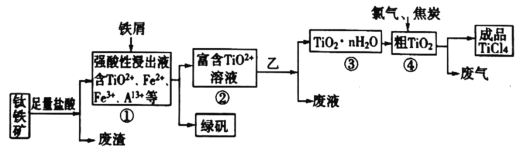
��Ŀ����
����Ŀ�����ݰ�����ԭ����ͭ�ķ�Ӧ������ƲⶨͭԪ�����ԭ������Ar��Cu�� (����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������m(H2O)���ɴ˼���Ar��Cu����Ϊ�ˣ��ṩ��ʵ���������Լ�����(������Ҫ���ظ�ѡ�ã������NH4C1��Ca(OH)2�������Բ���ʹCuO��ȫ��ԭ�İ���)��
��ش��������⣺
(1)������ԭ��������ͭ�Ļ�ѧ����ʽΪ________________________________��
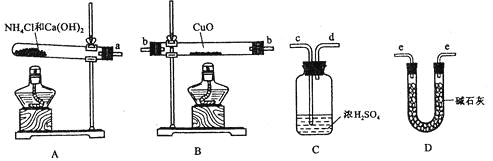
(2)�����ṩ���������Լ���ѡ����װ��ʵ���һ��������װ�ã����������������˳��Ϊ(��ͼ�б�ע�ĵ��ܿڷ��ű�ʾ)a��______________________________��
(3)�ڱ�ʵ���У������m(CuO)=ag��m(H2O)=bg����Ar��Cu��= _______________��
(4)�ڱ�ʵ���У�ʹ�ⶨ���Ar��Cu��ƫ�����_______________ (�����)��
��CuOδ��ȫ��Ӧ �� CuO������
��CuO�л��в���Ӧ������ �ܼ�ʯ�Ҳ�����
��NH4C1��Ca(OH)2����ﲻ����
(5)�ڱ�ʵ���У�����ͨ���ⶨ_______________��_______________����_______________��_______________�ﵽʵ��Ŀ�ġ�
���𰸡�2NH3+ 3CuO![]() 3Cu + 3H2O + N2 a�� e�� b�� e
3Cu + 3H2O + N2 a�� e�� b�� e ![]() -16 �٢� m(CuO) m(Cu) m(Cu) m(H2O)
-16 �٢� m(CuO) m(Cu) m(Cu) m(H2O)
��������
��1��������������ԭ�ԣ��ڼ��������¿��Ա�����ͭ������������Ϊ������ͭ��ˮ����Ӧ�Ļ�ѧ����ʽΪ2NH3+3CuO![]() 3Cu+3H2O+N2��
3Cu+3H2O+N2��
��2����Ϊ��Ҫ�ⶨ��Ӧ��������ˮ�����������Ա��豣֤ͨ��İ����Ǵ�������ģ�����Ũ��������백����Ӧ�����ֻ��ͨ����ʯ�ҽ��и����ͨ������ͭ���з�Ӧ�������ͨ���ʯ�����շ�Ӧ���ɵ�ˮ���Բ������ˮ��������������ȷ��˳��Ϊa��e��b��e��
��3�����ݷ�Ӧ����ʽ2NH3+3CuO![]() 3Cu+3H2O+N2��֪������ͭ��ˮ�����ʵ�����ȣ����������¹�ϵʽ��
3Cu+3H2O+N2��֪������ͭ��ˮ�����ʵ�����ȣ����������¹�ϵʽ��![]() =
=![]() �����Ar(Cu)=
�����Ar(Cu)=![]() ��16��
��16��
��4���ɣ�3����֪��Ar(Cu)=![]() ��16������CuOδ��ȫ��Ӧ��˵��bƫС�����ƫ����CuO�����˵��aƫС��bƫ���ƫ�ͣ�����CuO�л��в���Ӧ�����ʣ�˵��bƫС�����ƫ������ʯ�Ҳ����˵���������ﲻ���ף�bƫ���ƫ�ͣ�����NH4C1��Ca��OH��2����ﲻ�����ֻҪ�������ﳹ�ף��Խ����Ӱ�죻�ʴ�Ϊ�٢ۣ�
��16������CuOδ��ȫ��Ӧ��˵��bƫС�����ƫ����CuO�����˵��aƫС��bƫ���ƫ�ͣ�����CuO�л��в���Ӧ�����ʣ�˵��bƫС�����ƫ������ʯ�Ҳ����˵���������ﲻ���ף�bƫ���ƫ�ͣ�����NH4C1��Ca��OH��2����ﲻ�����ֻҪ�������ﳹ�ף��Խ����Ӱ�죻�ʴ�Ϊ�٢ۣ�
��5�����ݷ�Ӧ����ʽ2NH3+3CuO![]() 3Cu+3H2O+N2��֪��Ҳ����ͨ���ⶨm��CuO����m��Cu����m��Cu����m��H2O�����ﵽʵ��Ŀ�ģ��ʴ�Ϊm��CuO����m��Cu����m��Cu����m��H2O����
3Cu+3H2O+N2��֪��Ҳ����ͨ���ⶨm��CuO����m��Cu����m��Cu����m��H2O�����ﵽʵ��Ŀ�ģ��ʴ�Ϊm��CuO����m��Cu����m��Cu����m��H2O����